Highlights
- Artificial intelligence (AI) identified mitochondrial damage control molecules from a library of traditional Chinese medicinal plant compounds.
- Two of these molecules – Kaempferol and Rhapontigenin – reduce classic Alzheimer’s disease markers in worms and mice.
- Alzheimer’s disease mice fed Kaempferol and Rhapontigenin show improvements in spatial and visual memory.
Alzheimer’s disease (AD) treatments are a dime a dozen. Too many target the toxic neurofibrillary tangles and β-amyloid plaques that have characterized AD for so many years and have failed. Another treatment angle has been inducing mitophagy – the recycling of damaged mitochondria – because this process declines in neurodegenerative diseases like AD. However, mitophagy-inducing drug candidates fit to treat people with conditions like AD have been tough to come by.
A recent study published in Nature Biomedical Engineering demonstrates that artificial intelligence (AI) can find mitophagy-inducing compounds to treat AD. In a collaboration between scientists in China and Norway, Xie and colleagues implement AI to identify Kaempferol and Rhapontigenin as potential treatments for AD. Kaempferol and Rhapontigenin are common plant compounds that induce mitophagy.
Mitophagy Declines in Alzheimer’s Disease
Mitochondria – structures that provide energy to each cell they inhabit – are constantly damaged by typical cellular activity and routinely cleared by a process called mitophagy. Due to aging, mitophagy is reduced up to 50% in AD patients, leading to damaged mitochondria buildup. Getting rid of these mitochondria seems to help, as animal studies show that restoring mitophagy slows down the disease progression of AD.
Finding the Perfect Mitophagy Inducers
Traditional screening methods for finding new drugs is time consuming and labor intensive, so Xie and colleagues utilized AI to find the optimal mitophagy inducers. They used multiple biological databases to compile a dataset of 19.9 million compounds and used machine learning to train the AI to find structural similarities between compounds. 14 known mitophagy inducers were then compared to a library of 3,724 natural compounds isolated mainly from traditional Chinese medicinal plants to identify 18 potential mitophagy inducers.
The 18 mitophagy-inducing candidates were tested experimentally in human cells (HeLa cells) and worms (C. elegans), of which three induced mitophagy: Quercetin, Kaempferol, and Rhapontigenin. Although Quercetin has grown in popularity in anti-aging and longevity fields, it did not improve memory in worms and was not used further in the study.
Kaempferol and Rhapontigenin Improve Memory in AD Worms
One of the primary symptoms of AD and dementia is memory loss. To determine if inducing mitophagy in AD improves memory, Xie and colleagues used worms genetically manipulated to have either neurofibrillary tangles or β-amyloid plaques in their brain cells. To measure memory, the researchers exposed the worms to a chemical that the worms are aversive to (isoamyl alcohol), then checked if the worms remembered to stay away from the chemical.
Both types of AD worms (neurofibrillary tangle and β-amyloid plaque worms) had improved memory after being treated with Kaempferol and Rhapontigenin. In addition, the Chinese and Norwegian scientists showed that both Kaempferol and Rhapontigenin reduced neurofibrillary tangles. Furthermore, Kaempferol and Rhapontigenin had a neuroprotective effect on the worms, which, along with the reduction in neurofibrillary tangles may explain the improvements in memory.
Kaempferol and Rhapontigenin Improve Spatial and Visual Memory in AD Mice
Encouraged by the results they found in worms, Xie and colleagues wondered whether the anti-AD effects of Kaempferol and Rhapontigenin would translate to rodents, so they began experimenting with a mouse model for AD. To measure the spatial and visual memory of the AD mice, the researchers ran several tests, including the Morris water maze test and visual recognition test.
In the Morris water maze test, the mice were trained to find a platform in a pool of water. The researchers then removed the platform and counted the number of times the mice swam by where the platform used to be. Treatment with Kaempferol and Rhapontigenin increased the number of times the AD mice swam through where the platform used to be, indicating improvements in spatial memory.
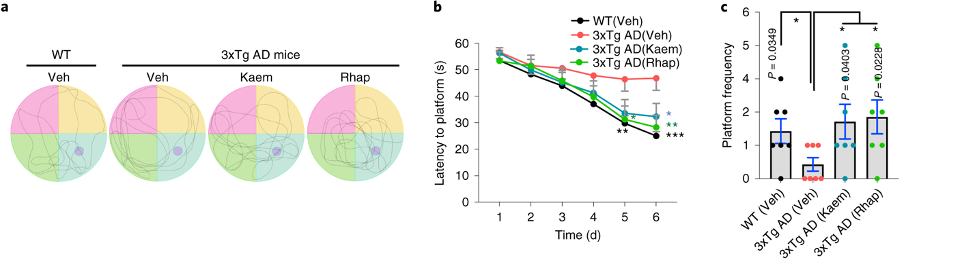
(Xie et al., 2022 | Nature Biomedical Engineering) Kaempferol and Rhapontigenin Improve Spatial Memory in AD Mice. (a and c) AD mice (3xTg) passed through the former platform location (pink dot in the blue quadrant) more frequently when treated with Kaempferol and Rhapontigenin. (b) Kaempferol and Rhapontigenin treated AD mice find the platform in less time than untreated mice after training for four days.
To measure visual memory in the AD mice, the researchers placed the mice in a box to explore two objects. The mice were then returned to the box but with one object replaced with a new one. Since mice like to explore new objects, which requires remembering past encounters, more time spent exploring the new object over the old one reflects better visual recognition and memory. Treatment with Kaempferol and Rhapontigenin increased the time spent exploring the new object, indicating improved visual memory for the AD mice.
Kaempferol and Rhapontigenin Reduce Pathological Aggregates and Increase Mitophagy
Xie and colleagues next determined whether the improvements in memory observed in the AD mice were due to the alleviation of neurofibrillary tangles and β-amyloid plaques. They found that Kaempferol and Rhapontigenin reduced the neurofibrillary tangles and β-amyloid plaques in a region critical for learning and memory, the hippocampus, of the AD mice.
The international researchers also found that deficits in mitophagy-related proteins in the AD mice could be restored by Kaempferol and Rhapontigenin, and that the accumulation of mitochondria could be cleared in the brains of the AD mice. Together, these findings suggest that the improvements in memory observed in the AD mice were due to an increase in mitophagy and reduction in AD aggregates.
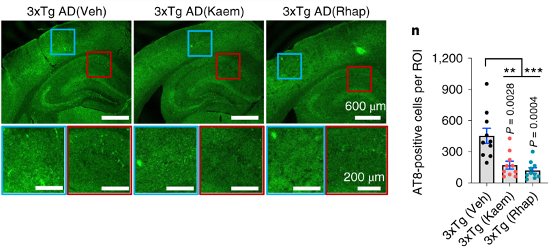
(Xie et al., 2022 | Nature Biomedical Engineering) Kaempferol and Rhapontigenin Reduce Neurofibrillary Tangles and β-amyloid Plaques in AD Mice. AD mice have fewer neurofibrillary tangles (visualized with AT8) when treated with Kaempferol and Rhapontigenin.
Kaempferol and Rhapontigenin as a Promising Treatment for AD
Xie and colleagues showed that memory could be improved in models for AD by increasing mitophagy with Kaempferol and Rhapontigenin. These findings suggest that Kaempferol and Rhapontigenin can potentially treat AD in humans, especially since there were no detectable side effects. Also, what possibly makes Kaempferol and Rhapontigenin more powerful is that they act on both neurofibrillary tangles and β-amyloid plaques, whereas other drugs usually target only one of these AD aggregates.
Plant-based compounds like Kaempferol and Rhapontigenin seem like promising treatments for AD. A human study suggests that plant molecules called flavonols like Kaempferol reduce the risk of AD. The anti-oxidant and anti-inflammatory properties of Kaempferol are also auspicious. Rhapontigenin has similar effects to Kaempferol and is an analog to resveratrol, which has shown anti-aging and neuroprotective properties. Like resveratrol, Rhapontigenin is found in grapes, and Kaempferol is found in beans, tea, kale, and broccoli.
AI is the Future of Drug Discovery
Numerous heavy investments and business deals have been made surrounding AI and drug discovery. Last year, an anti-cancer drug developed by the German biotechnology company Evotec made it to clinical trials. The design of this drug would have taken 4- 5 years, but thanks to AI it took only eight months. Andrew Hopkins, the CEO of Exscientia, the AI company that partnered with Evotec to develop the drug, says that soon AI will be part of developing every new drug. Researchers can utilize the AI approach to drug discovery to treat everything from kidney disease to amyolateral sclerosis (ALS).
The authors point out that AI-driven drug screenings have a higher success rate than traditional non-AI screenings. In the future, when more reference compounds are available, researchers can use AI to find new, structurally different molecules. From this perspective, when properly manned, AI can push the biomedical field forward in the right hands.