The molecule nicotinamide adenine dinucleotide (NAD+) plays a crucial role in a multitude of biological reactions that generate cellular energy, repair DNA, and maintain cellular health. Moreover, it drives the activation of NAD+-consuming enzymes, including sirtuins, poly (ADP-ribose) polymerases (PARPs), and cluster of differentiation 38 (CD38). Importantly, studies show that NAD+ declines with age and is associated with age-realted diseases, such as cardiovascular, neurodegenerative, and metabolic conditions. For these reasons, researchers continue their quest to uncover how maintaining NAD+ levels during aging mitigates age-related diseases along with its roles in cultivating cellular health.
NAD+’s Role in Cell Energy Production
From bacteria to primates, NAD+ plays critical roles in cell metabolism. This essential molecule functions as a cellular shuttle bus, transporting electrons from one biological molecule to the next within cells. Along with its molecular counterpart, NADH (an NAD+ molecule with an added hydrogen atom), NAD+ transfers electrons to drive the production of adenosine triphosphate (ATP), the cell’s “energy” molecule. What’s more, studies have correlated reduced NAD+ levels in age-related diseases with inhibited ATP synthesis.
NAD+ Activates Enzymes that Maintain Cell Health
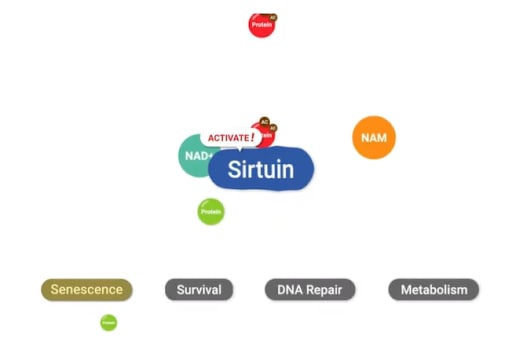
NAD+ activates enzymes called sirtuins, which modulate cellular metabolism, keep chromosomes stable, repair damaged DNA, and reduce cellular stress. Most sirtuins carry out their cell maintenance functions by removing cellular tags called “acetyl groups” from other cell proteins to activate them. Seven types of sirtuins carry out their functions within different cellular compartments, localized to the cell’s nucleus, the cell’s aqueous interior (the cytoplasm), or the cells’ power-generating structure (the mitochondria).
Sirtuins1-7 play various, interrelated roles in maintaining cellular health and possibly mitigating the aging process when adequately activated. Localized to the cell’s nucleus and cytoplasm, Sirtuin1 regulates metabolism and tissue inflammation. Sirtuin2, also localized to the nucleus and cytoplasm, plays roles in regulating cell proliferation and inhibiting tumor development. Sirtuin3 is located in mitochondria and also mediates cell metabolism. Also localized to the mitochondria, Sirtuin4 regulates insulin secretion. Sirtuin5 functions within the mitochondria to detoxify cells from harmful waste products like ammonia. Sirtuin6 carries out its DNA repair and metabolism regulation tasks within the cell’s nucleus. Finally, within the nucleus, Sirtuin7 regulates cell protein generation by mediating the production of ribosomes – tiny units of ribonucleic acid (RNA) that synthesize proteins.
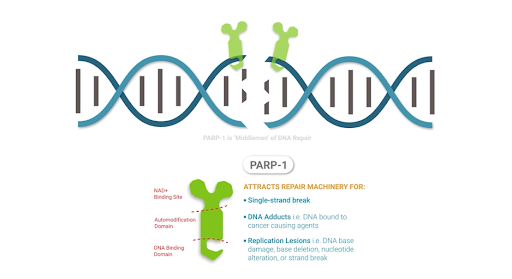
Another major class of enzymes that NAD+ activates are poly (ADP-ribose) polymerases (PARPs), which play key roles in DNA repair, chromosomal stability, and programmed cell death. PARPs localize to the cell’s nucleus and initiate responses to DNA breaks from harmful metabolic conditions, chemicals, or radiation. Once PARPs detect a DNA breakage, they bind to DNA and begin the synthesis of a new chain of DNA with the help of other DNA-repairing enzymes. PARPs require NAD+ to generate the new DNA strands.
Overactivation of PARPs from excessive DNA damage may encompass a significant source of NAD+ depletion, which can induce the progressive loss of cell energy (ATP). This scenario often results in cell aging and death. Restoring NAD+ levels with supplementation may provide a means to restore cellular energy and overcome PARP-induced NAD+ depletion.
Another important NAD+-consuming enzyme is CD38. CD38 enzymes are found on the surfaces of many types of white blood cells. A gradual increase in CD38 levels throughout the body has been linked to declining NAD+ levels with age. Treating aged mice with the pharmaceutical 78c, a CD38 inhibitor, has been shown to inhibit this age-related NAD+ decline. Moreover, aged mice with deleted CD38 exhibit twice the NAD+ levels than their healthy, aged counterparts. On the other hand, aged mice with genetic alterations which cause them to have too much CD38 display drastically reduced NAD+ levels and have mitochondrial dysfunction. Since mitochondrial dysfunction is linked to most age-related diseases, this suggests that CD38 plays a critical role in age-related NAD+ depletion and disease onset.
Ways to Maintain NAD+ Levels
From energy production to DNA repair, NAD+ plays critical roles in numerous processes that facilitate cellular health. Falling NAD+ levels with aging may therefore spawn the accumulation of DNA mutations and lead to cell death. Finding new ways to maintain NAD+ levels as we grow older to prevent age-related diseases has become a paramount endeavor. For this purpose, people have begun taking NAD+ precursors like nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR), which studies have shown to increase blood NAD+ concentrations. What’s more, supplementation with NAD+-consuming enzyme inhibitors like the pharmaceutical 78c may be on the horizon as a new way to increase NAD+ levels.
The future will tell whether increasing and maintaining NAD+ levels as we age extends lifespan and prevents age-related diseases. However, tantalizing evidence from rodent studies suggests that humans may at least ward off age-related diseases by doing so to add onto the number of healthy years they live.