- History of Gene Therapy
- Gene Therapy Strategies
- Targeted Genes for Gene Therapy
- Safety Concerns for Gene Therapy Make Future Research Essential
The natural process of aging engenders physiological decline, including the precipitation of age-related cardiovascular, metabolic, and neurodegenerative conditions, along with cancer. Since many age-related diseases stem from single or multiple mutations, therapies targeting genes — gene therapies — have received more and more attention in recent years.
History of Gene Therapy
In the 1960s, Marshall Warren Nirenberg first proposed genetic engineering applications that would make gene therapy possible. More recently, in the 21st century, various gene therapy technologies have been created and developed, especially the clustered regularly interspaced short palindromic repeats (CRISPR) technology. Such technologies not only allow editing, such as knocking out genes, but also epigenetic modifications, where what proteins expressed are altered without altering genetic information. These gene therapy technologies have allowed more precise regulation of protein production from genes. The application of gene therapies and their abilities to influence the activity of target genes against aging and age-related diseases may lead to profound healthcare technology in the future.
Gene Therapy Strategies
Gene therapy strategies include gene replacement, gene editing, and epigenetic modification. The typical means to deliver genes use viruses containing engineered DNA sequences that incorporate into targeted cells along with fat (lipid) nanoparticles delivering the gene editing DNA sequence. The gene therapies encompass not only gene sequence editing but also modifications of the RNA produced from DNA, which then translate to proteins in cells.
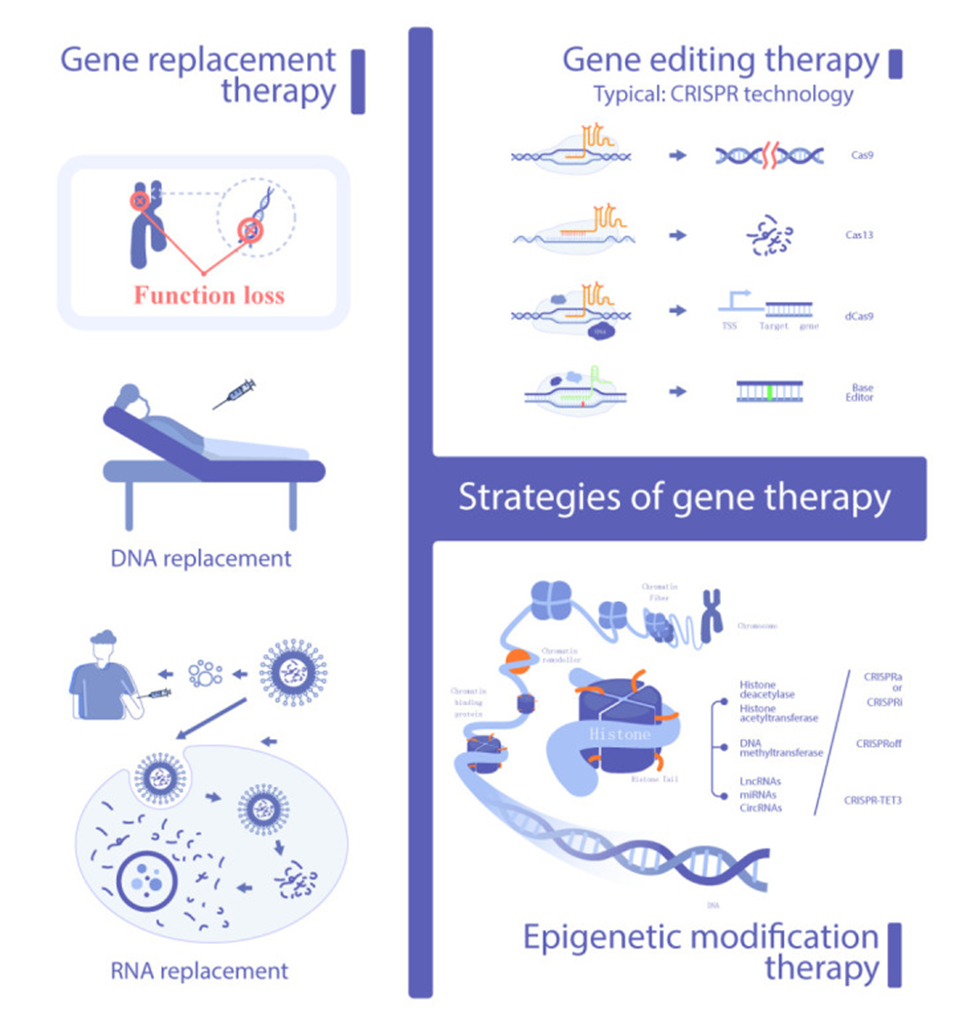
Targeted Genes for Gene Therapy
As research on age-related genes continues to improve, an increasing number of aging-related genes have been identified. As such, there could be hundreds of aging-related genes to be discovered in the future, exhibiting positive and negative effects toward aging. Moreover, researchers have found that numerous genes involved in aging-related diseases overlap with longevity-associated genes, which suggests that targeting aging-related genes may have great therapeutic potential against age-related conditions.
Telomerase gene
Telomeres are at the ends of chromosomes, which are essential to protect and maintain the length of chromosome ends and preserve chromosomal stability and function. Telomeres are shortened through DNA replications along with wear and tear but can be extended by the telomerase enzyme. Numerous studies have shown that defective telomeres or telomerase enzymes exert substantial contributions to aging-related diseases. Thus, targeting the gene coding for telomerase — the telomerase gene — could be a way to mitigate aging-related diseases and improve health as we grow older.
In 1999, using cells in laboratory dishes, Bodnar and colleagues inserted a telomerase gene (TERT) into human blood vessel cells (endothelial cells) with a viral vector. In doing so, the researchers showed evidence of delayed cellular aging and elongated telomeres. Although this study was performed with cells and not a living organism, it provides evidence that targeting the telomerase gene with gene therapy could serve as a means to counter the effects of aging.
Further studies using mice showed positive benefits from TERT gene therapy against neurodegenerative disorders. For example, Wan and colleagues found that increasing TERT activation induced improved motor function against Parkinson’s disease. Furthermore, no cancerous tumors associated with this therapy were found. These findings serve as a basis for further research using telomerase gene therapy in clinical trials.
APOE
APOE is a gene that researchers currently recognize as having a robust association with human longevity. The protein it encodes, apolipoprotein E, is a pivotal regulator of fat (lipid) metabolism and is abundantly distributed in the liver, kidney, fat, and brain. Certain mutations in the APOE gene are risk factors for Alzheimer’s disease, while other mutations in APOE are protective against Alzheimer’s disease. Thus, APOE could present a promising target for gene therapy against aging and aging-associated diseases like Alzheimer’s disease. Along those lines, a clinical trial is currently underway to find whether gene therapy using viral vectors that target APOE benefits humans with Alzheimer’s disease.
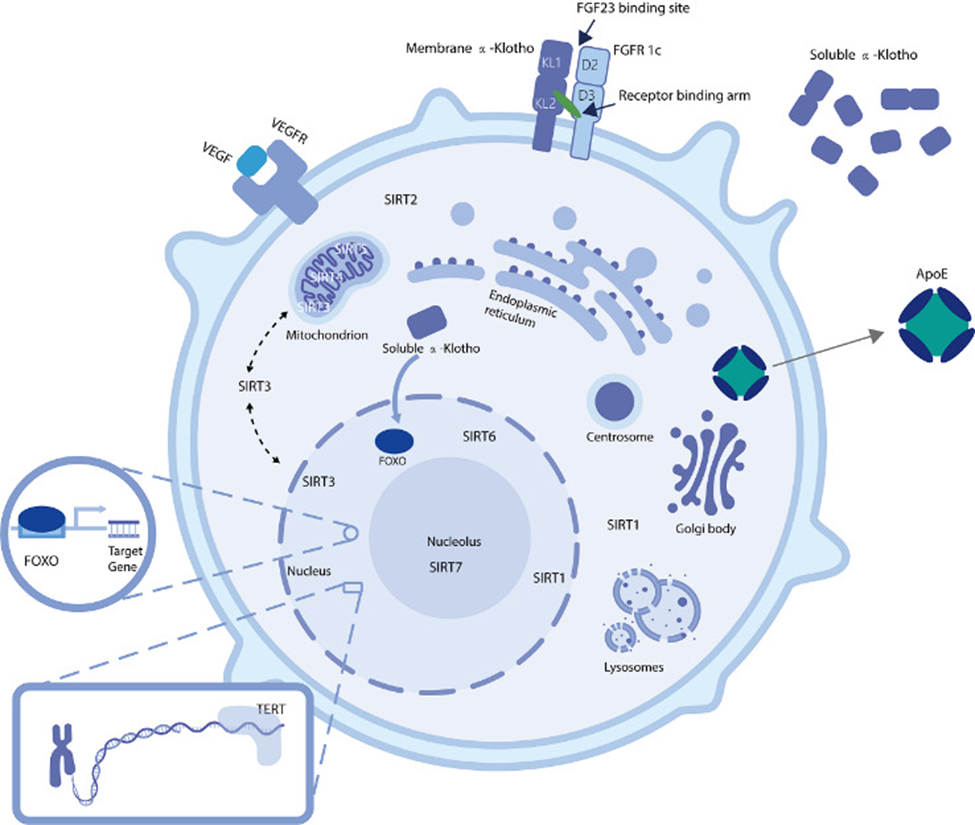
KLOTHO
KLOTHO is an aging-related gene well known throughout the aging research field. In 1997, Japanese scientists Kuro-o and colleagues reported mutant mice with a KLOTHO gene deficiency exhibited premature aging. Restoring KLOTHO activation delayed the mice’s aging and extended their lifespans up to 30%. These results have made KLOTHO research enticing since this study strongly linked its gene activity to longevity in rodents.
KLOTHO encodes proteins involved in suppressing inflammation. Inflammation is known to play key roles in aging processes and age-related diseases. Along those lines, other mouse studies have shown boosting KLOTHO activity with gene therapy improves neurodegenerative diseases, kidney diseases, and cardiovascular diseases. Currently, no clinical trials are underway to find whether KLOTHO gene therapy works in humans.
FOXOs
The forkhead box sub-group O (FOXO) gene was first shown to influence longevity based on analyses of long-lived Japanese individuals between 95 and 100 years old. The protein family it encodes are activated under stressful conditions, including starvation, energy deficiency, and DNA damage. Under these circumstances, the FOXO proteins bind to DNA as transcription factors that influence DNA activation. In doing so, they regulate gene activity for cell death, cell metabolism, antioxidants, and cell waste removal and recycling (autophagy). As such, the FOXO genes are promising targets for gene therapy against aging and age-related conditions.
Neurodegenerative disease research suggests FOXO genes have down regulated activity resulting in cognitive and motor function impairment, contributing to Alzheimer’s disease and Parkinson’s disease progression. Targeting FOXO genes with gene therapy may alleviate age-related neurodegenerative diseases in future research, and mouse model research examining FOXO’s functions is underway.
SIRT
Humans have seven sirtuins (SIRT1-7). Sirtuins function to initiate DNA repair and alleviate cells of unstable and deleterious molecules called reactive oxygen species. Along those lines, these genes and the proteins they encode have been tied to aging and age-related diseases.
Delivering SIRT7 with a viral vector has been shown to extend the lifespans of mice with a condition called progeria where they age prematurely. Further research on SIRT gene therapy is expected to find whether this technique increases lifespan and delays aging-related diseases.
VEGF
The vascular endothelial growth factor (VEGF) gene encodes proteins involved in the formation and function of blood vessels. High levels of VEGF activation have been linked to protective effects on the cardiovascular system. Other studies have shown that it could be a promoting factor for malignant tumors. Due to these conflicting findings, more studies are necessary to determine if VEGF gene therapy could benefit longevity by conferring protection to the cardiovascular system.
Safety Concerns for Gene Therapy Make Future Research Essential
Gene therapy entails gene replacement, gene editing, functional RNA replacement, and epigenetic modifications. Using these methods to replace, edit, or modify gene activation typically involves using delivery mechanisms like viral vectors or lipid nanoparticles.
Safety concerns surrounding gene therapy include the potential to promote cancerous growths by altering gene activity. For this reason, a number of clinical trials will need to be completed before the prospect of undergoing gene therapy in a clinic comes to fruition.