- Why Do We Age?
- Reversing Aging with Cellular Reprogramming
- When will Cellular Reprogramming Reach Consumers?
Why Do We Age?
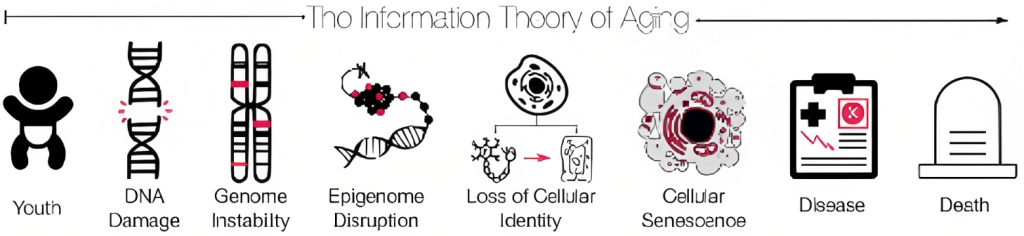
Scientists are unable to agree upon the biological reasons for aging, but there are few theories. One such theory is the Information Theory of Aging proposed by Harvard professor David Sinclair. The theory states that aging is caused by a loss of information. This information is lost due to the dysregulation of how the genes stored within our DNA are activated.
Recently, a study done by Sinclair and his colleagues provided evidence supporting the Information Theory of Aging. In the study, aging was induced by double-strand breaks in DNA. This specific type of DNA damage is thought to cause instability of the genome — all the possible genes that can be activated in a cell — and disrupt the epigenome — the molecules that control the activation of genes in the genome.
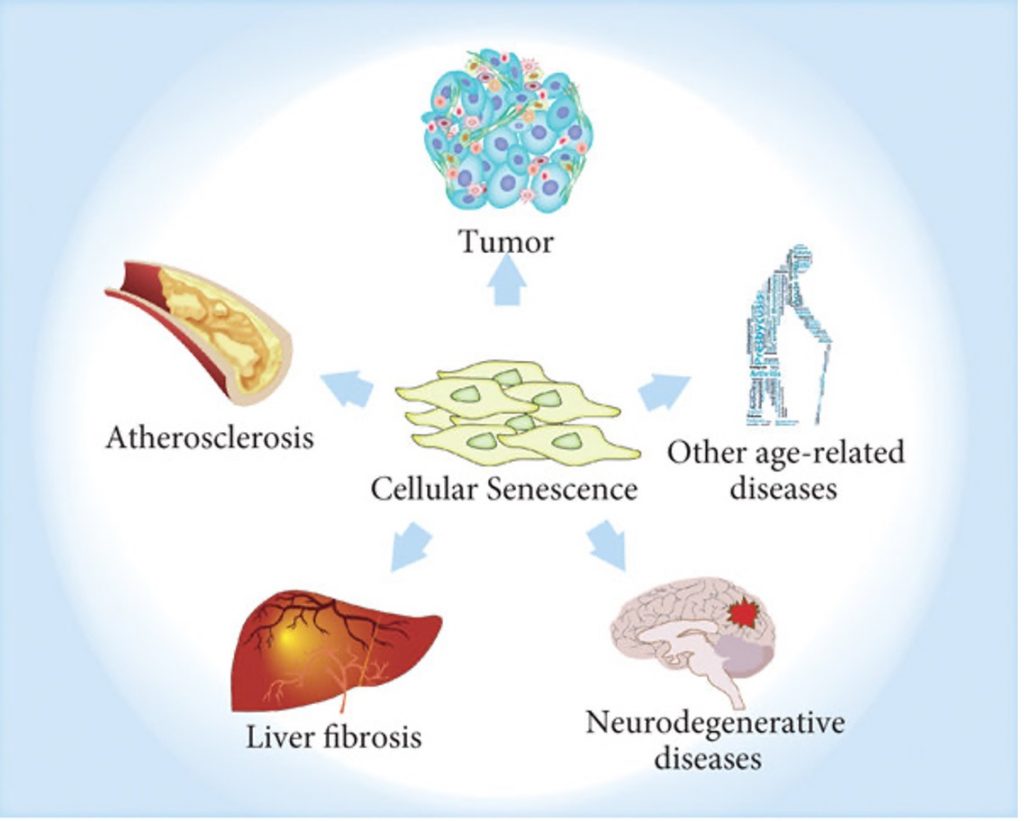
According to the theory, the dysregulation of gene activity that comes with genomic instability and epigenome disruption triggers a loss of cellular identity. This is because the genes activated within a given cell at a given time determine how a cell functions, and thus determine its role or identity in the body. Eventually, this loss of identify causes the cell to become senescent.
Senescent cells appear to drive the aging process by promoting chronic diseases with high risk of mortality, such as cardiovascular disease. Thus, the Information of Theory of aging posits that aging begins at the level of DNA, leading to changes at the levels of the cell in the form of senescence, until finally manifesting in disease, and ultimately death.
Reversing Aging with Cellular Reprogramming
Japanese scientist Dr. Shinya Yamanaka won the Nobel Prize in Physiology or Medicine in 2012 for discovering how to turn normal mouse cells into stem cells. He did so by forcibly activating a set of four genes (Oct3/3, Sox2, Klf4, and c-Myc) now called “Yamanaka factors.” It wasn’t too long before this cellular reprogramming technology was applied to age reversal experiments.
How Does Cellular Reprogramming Work?
Biological Clocks
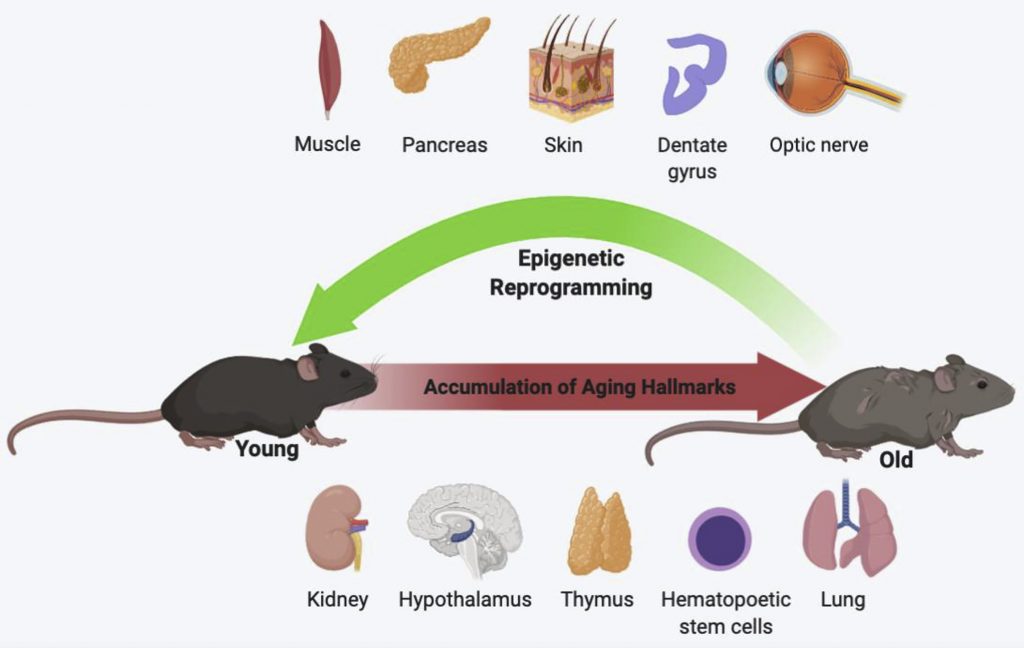
Both the Salk Institute and Rejuvenate Bio have shown that cellular reprogramming reverses the biological age of mice. Biological age is measured by various biomarkers and can accurately predict chronological age in humans.
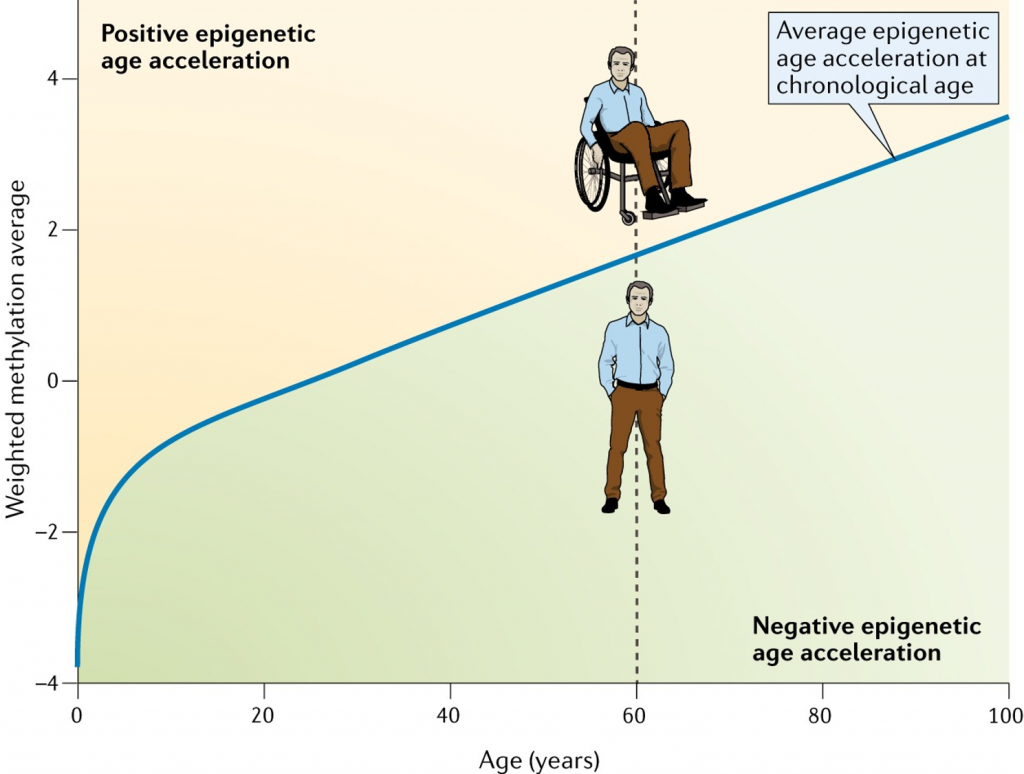
When an individual’s biological age is higher than their chronological age, we can say their age has been accelerated. When their biological age is lower than their chronological age, we can say their age has been reversed.
Over a similar timeline as cellular reprogramming’s discoveries, aging clocks have been developed and utilized to estimate biological age. These biological clocks are often called epigenetic clocks.
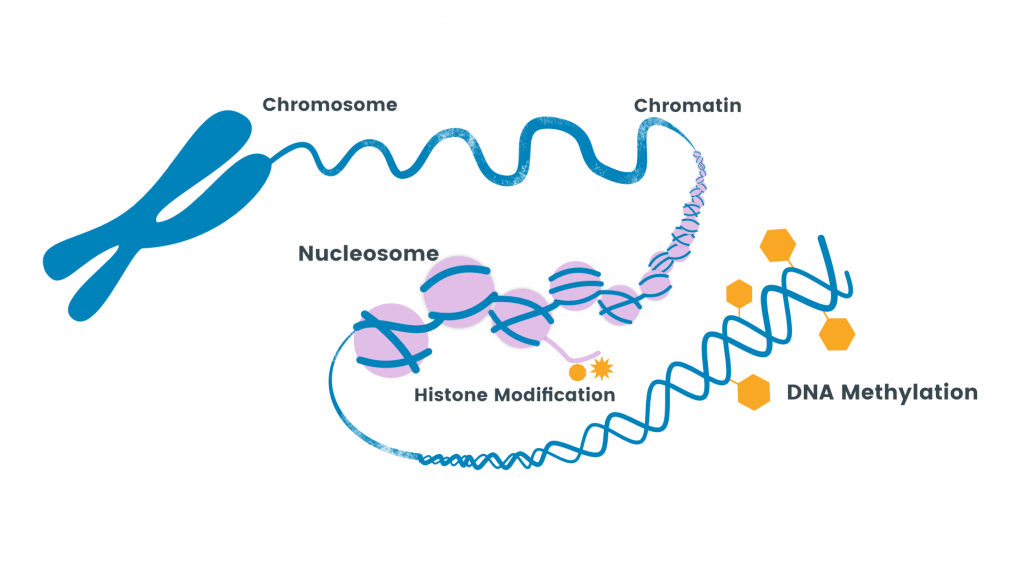
Epigenetics is a phrase used to describe how our genes can be turned on or off without making changes to our DNA sequence. One form of epigenetic regulation is DNA methylation, which involves chemical tags called methyl groups attached to specific regions of DNA. Epigenetic clocks are based on the placement of these chemical tags throughout our DNA, which changes with aging.
Understanding epigenetic clocks can help with understanding cellular reprogramming and possibly even aging itself if the Information Theory of Aging is accurate. When a methyl group is added or removed from a specific region of DNA, it changes which genes are turned on or off. Whatever genes are turned on or off in a given cell determines how the cell operates. The DNA methylation patterns associated with aging tend to cause cells to operate poorly, leading to poor outcomes like disease.
Scientists from the Babraham Institute in the U.K. showed that cellular reprogramming reverses the epigenetic age of human skin cells by thirty years. Furthermore, the rejuvenated skin cells resembled young cells and produced more collagen, the protein that makes our skin hydrated and firm but declines with age. This study demonstrates the association between biological age and cellular rejuvenation.
Partial Epigenetic Reprogramming
How does cellular rejuvenation through cellular reprogramming work? The Yamanaka factors used for reprogramming are called “factors” because they are transcription factors. Transcription factors are proteins that initiate the activation of genes — they turn genes on. Yamanaka factors work by turning on genes that tell a cell to become a stem cell (induced pluripotent stem cell).
A stem cell is like a baby cell with no cellular identity that is capable of becoming any cell type, like a neuron or muscle cell. While the details are still being worked out, reprogramming adult cells into stem cells seems to erase the epigenetic changes that occur with aging. One way this occurs is by changing where methyl groups are attached to DNA, changing which genes are turned on or off. This change in DNA methylation also turns back the epigenetic clock.
However, fully reprogramming a cell back into a stem cell isn’t the goal with age reversal experiments. The goal is to activate the Yamanaka factors just long enough to reverse epigenetic aging without changing cellular identity. This way, a neuron remains a neuron, albeit a neuron with reduced epigenetic age. Thus, partial epigenetic reprogramming is the more precise term to use and it’s analogous to hacking DNA to revert cells back to an earlier stage of development.
Delivering Yamanaka Factors via Gene Therapy
How can Yamanaka factors be delivered to human cells? The technique used by Rejuvenate Bio to extend the lifespan of naturally aged mice and by Life Biosciences to restore vision in primates was a form of viral-mediated delivery. This method involves using the delivery system used by viruses, which have evolved the machinery to effectively deliver their genes to our cells.
Rejuvenate Bio and Life Biosciences specifically used adeno-associated virus (AAV)-mediated delivery. AAV-mediated delivery is preferred because it elicits less of an immune response compared to other viral-mediated delivery systems. To do this, the viral genes are removed and then replaced with Yamanaka factors. The Yamanaka
How Effective is Cellular Reprogramming?
Reprogramming Extends Mouse Lifespan
Salk Institute scientists were the first to show that Yamanaka factors are capable of extending the lifespan of animals. In the groundbreaking 2016 study, the California-based researchers showed that cellular reprogramming could increase the lifespan of progeria mice by 20%.
Progeria mice model a human disease called Hutchinson-Gilford progeria syndrome, which causes premature aging. The Salk researchers bred progeria mice with mice genetically modified to carry Yamanaka factors, resulting in prolonged lifespan. Reprogramming also reversed signs of aging like elevated heart rate and skin thinning.
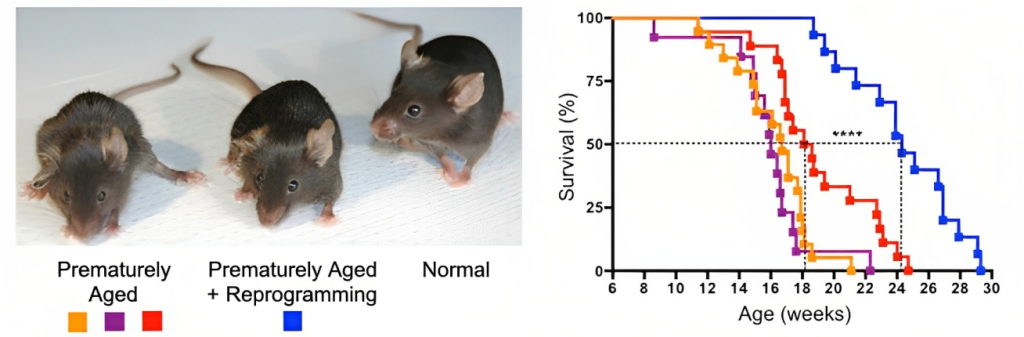
The 2016 Salk Institute study was replicated in 2022 when French scientists extended the lifespan of progeria mice by 15%. In addition to reversing signs of bone and skin aging, the French scientists found that reprogramming increased the grip strength of the mice, a sign of muscle age reversal. Notably, the mice used by the French researchers had a less aggressive form of progeria and lived about 15 weeks longer than the Salk Institute progeria mice.
Following the progeria mice studies, in early 2023, Rejuvenate Bio was the first to extend the lifespan of naturally aged mice with reprogramming. Because all four Yamanaka factors had been shown to cause tumor growth in normal mice, the Rejuvenate Bio researchers used only three (Oct3/3, Sox2, Klf4 [OSK]). Importantly, they used gene therapy methods to deliver the Yamanaka factors, technology that has already been used on humans (with other genes).
Reprogramming Reverses Aging
In a landmark 2020 study led by Dr. David Sinclair at Harvard, cellular reprogramming was used to restore vision in mice. In this study, the OSK gene therapy method (similar to Rejuvenate Bio’s method) was utilized. More recently, in 2023, it was revealed that this method restores vision in nonhuman primates. Based on the success of these studies, Sinclair has stated that the next step is restoring vision in humans.

Along with reversing vision loss, cellular reprogramming has the potential to reverse many diseases. Salk Institute scientists have reversed signs of aging in the kidney of naturally aged mice, which could lead to a reversal of kidney disease. Scientists from the Max Planck Institute in Germany have even reprogrammed heart cells to prevent damage from heart attacks. Such findings have implications for the reversal of heart disease.
When Will Cellular Reprogramming Reach Consumers?
The studies mentioned above have given investors a reason to spend their money. Several biotechnology companies are now working with cellular reprogramming technology, including Google’s Calico and Altos Labs, reportedly supported by Amazon CEO Jeff Bezos. More recently, the founder of ChatGPT Sam Altman made a $180 million investment in Retro Biosciences, a company devoted to extending the human lifespan using cellular reprogramming as one of their focus areas.
“Given the current trajectory of rejuvenation biotech, the pace of new discoveries in this field, and the amount of capital coming into this sector, rejuvenation science and biotech will most likely explode in the coming years.” – Dr. João Pedro de Magalhães and Dr. Alejandro Ocampo, leading experts in the field of epigenetic aging.
That being said, there is a lot of work to be done before cellular reprogramming can be tested in humans. One major obstacle will be to prevent the induction of cancer by targeting only specific groups of cells in the body. Additionally, petri dish (in vitro) studies have shown that reprogramming only works on some cells. Therefore, the effectiveness of cellular reprogramming will have to be optimized.
In another era, the outlook for cellular reprogramming in humans would seem like a therapy of the distant future. However, it was only in 2006 that Yamanaka and Takahashi showed that four transcription factors can reverse a cell’s epigenetic trajectory. At this rate, we may see cellular reprogramming being tested in humans within the next decade.