Key Points:
- 10 mg/week of rapamycin increases the lean mass of women by over 5%.
- The same dose reduces the pain of women, as measured by a survey.
Modern human society and medical technology have negated many of natural selection’s effects, allowing more people to live longer. However, an epidemic of poor lifestyle choices, along with the pollutants manufactured by modern society, perpetuate chronic conditions such as lung cancer, cardiovascular disease, and dementia. Thus, while we tend to live longer, many individuals tend to suffer from these chronic conditions, referred to as age-related diseases.
The goal of translational geoscientists is to test whether aging interventions can counteract age-related diseases and promote longevity in humans. Some of these aging interventions are simply existing drugs that are now being tested against aging, the most popular of which is rapamycin. Originally approved for the prevention of organ transplant rejection, rapamycin has proved to be one of the most potent aging interventions, at least in animal studies.
Adding to a growing body of clinical evidence revealing rapamycin’s age-intervening effects on humans, scientists at AgelessRx have found that the drug increases lean mass and reduces pain in older women but not older men. Importantly, long-term use of rapamycin taken once per week was shown to be safe for both women and men.
mTOR Inhibitor Increases Lean Mass and Reduces Pain
A total of 114 older adults participated in the study: 40 received 5 mg/week of rapamycin, 35 received 10 mg/week, and 39 received a placebo. Adverse effects attributed to rapamycin were limited, gastrointestinal distress being the only complaint more frequently reported in rapamycin groups than the placebo group.
Lean Mass
Aging tends to bring adverse changes to the composition of our body, like increased body fat and reduced muscle mass. To assess rapamycin’s effect on these changes, the researchers scanned participants with DXA (Dual-energy X-ray Absorptiometry), which measures visceral fat, bone mineral density, and lean mass (includes muscle, skin, and connective tissue). No significant changes in bone mineral density or visceral fat were observed in individuals taking rapamycin, but men in the placebo group gained visceral fat. Moreover, the results showed that lean tissue mass was increased in women who took 10 mg of rapamycin per week.
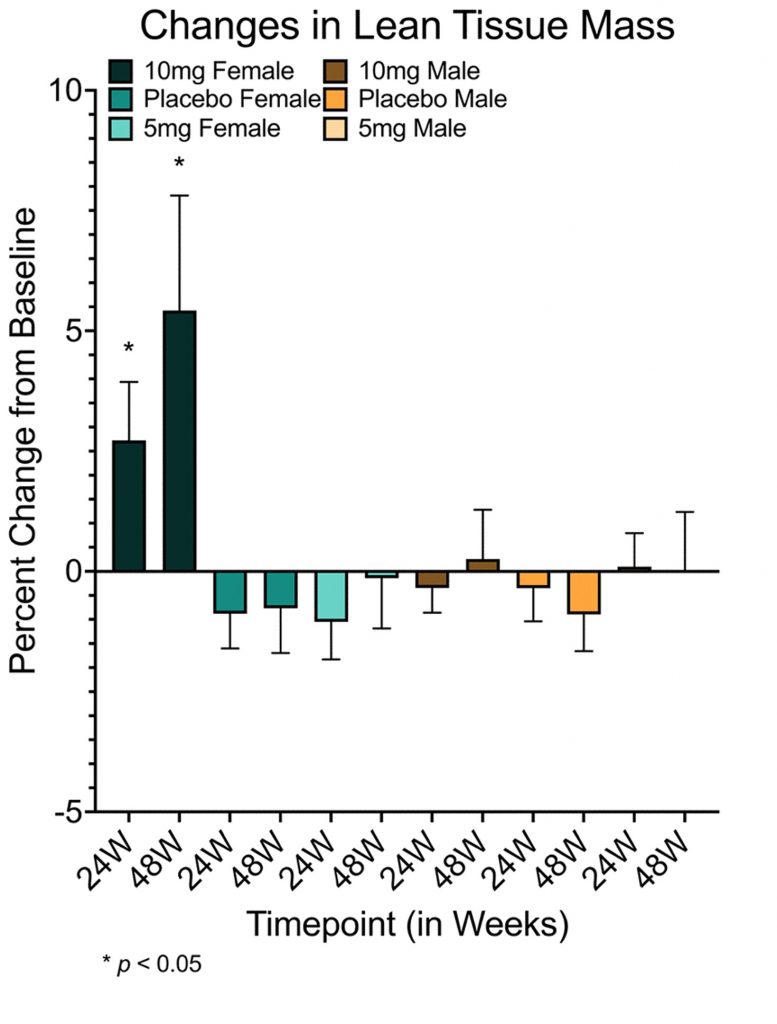
Pain
Reduced lean tissue mass is associated with poor quality of life, increased pain, and limited mobility, especially in older women. To evaluate the effect of rapamycin on quality of life, the researchers administered two surveys. Results from the WOMAC (Western Ontario and McMaster Universities Osteoarthritis) survey, which includes questions pertaining to pain, stiffness, and physical function, showed no significant changes. However, the SF36 (Short Form 36) survey, consisting of 36 quality of life questions, revealed that rapamycin leads to a reduction in perceived pain for the women who took 10 mg of rapamycin per week.
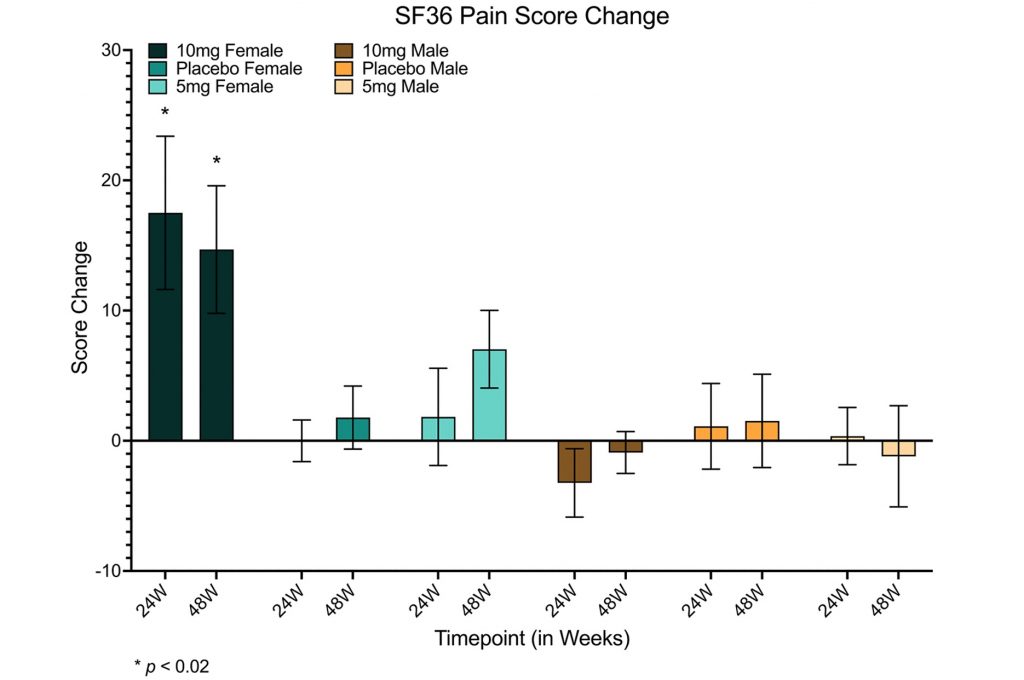
Additionally, general health improved in all participants taking 5 but not 10 mg/week of rapamycin, according to the SF36 survey. Moreover, in the 5 mg/week rapamycin group and placebo group, the SF36 survey showed that emotional well-being was improved after 48 weeks. These qualitative measurements will require quantitative evidence to improve validity.
Can mTOR Inhibitors Improve Lives?
Rapamycin was first shown to prolong the lifespan of mice in 2009 by inhibiting mTOR, an enzyme that signals cells to grow in the presence of abundant nutrients, especially protein. Inhibiting mTOR has since been shown to prolong the lifespan of mice with varying genetic backgrounds, as well as other organisms. However, whether inhibiting mTOR can prolong the lifespan of humans remains an open question.
Scientists have just begun to translate their studies of aging mice and other animals to aging humans. In the case of rapamycin, larger studies will help to confirm its efficacy in slowing human aging. In a recent study, off-label users of rapamycin reported,
“Improvements in health, happiness, brain function, feelings of youthfulness, confidence, calmness, anxiety, and generalized aches and pains. Interestingly, greater than fivefold more rapamycin users agreed with the comment that “family/friends have commented that I look good” than disagreed, suggesting that these perceived self-benefits may also be apparent to others.”
Most off-label users take rapamycin once per week, which is thought to reduce its immunosuppressive effects. This stems from a study showing that either 0.5 mg/day or 5 mg/week of rapamycin enhanced the immune system of older adults. The most frequent dose reported by the off-labels users was 6 mg with some going as high as 20 mg/week. Determining the optimal dose of rapamycin will require further research.
However, based on the findings of AgelessRx, 10 mg/week could be more effective than 5 mg/week, particularly for women. With that being said, taking 10 mg/week of rapamycin for up to 48 weeks appears to be safe.