Key Points:
- SIRT6 therapy has the potential to increase the lifespan of humans, but controversy surrounds how to activate sirtuins like SIRT6.
- SirTLab will embrace existing technologies with the help of world-renowned experts in the field.
- Because of the FDA’s outdated rules, SirTLab will start by treating conditions like fatty liver disease.
Countless animal studies have given credence to our newfound ability to delay aging and chronic disease with just a pill. The only problem is that many of these longevity interventions have yet to be tested on humans. Now Israel-based SirTLab Corporation is taking a huge step forward in solving this conundrum by planning to test SIRT6 therapies on humans.
SIRT6 belongs to the sirtuin family of seven enzymes, each of which uniquely promotes longevity. However, only SIRT1 (sirtuin 1) and SIRT6 (sirtuin 6) have achieved the holy grail of longevity medicines — extending the lifespan of mice. With grail in hand, the long-term goal of boosting SIRT6 is to extend our lifespan. Until we get there, SIRT6 has more to offer. The co-founder and CEO of SirTLabs, Boaz Misholi explains:
“It appears that high levels of SIRT6 also boosts physical performance and improves memory and cognitive function – even in young mice. But, while the longevity and healthspan benefits of SIRT6 are well-known, no one has yet developed a therapeutic way to increase SIRT6 levels at a cellular level in the body. Until now.”
Controversy Surrounding SIRT Boosters
As Misholi points out, activating SIRT6 in humans has never been done before. On top of that, controversy surrounds just how to activate sirtuins in the first place.
David Sinclair recently tweeted:
“Human trials with longevity molecules that directly activate Sirtuins will begin again in 6 months, 13 years after bad science from Pfizer almost killed my lab and the entire field. Onward!”
It was Sinclair and his team at Harvard who initially discovered that resveratrol activates SIRT1. Resveratrol is a chemical found in vegetables and fruits like grapes that began the “healthy” red wine drinking craze. In his tweet, Sinclair refers to a 2010 study by Pfizer concluding that resveratrol does not activate SIRT1.
The science battle continued in 2013 when Sinclair and his colleagues released a paper reaffirming that resveratrol and other synthetic compounds activate SIRT1. Sinclair even co-founded a company called Sirtris based on this idea.
“I think that the resveratrol experiments as well as our synthetic compounds … clearly puts to rest a lot of the controversy, well, ultimately all of the controversy regarding whether these compounds are directly activating SIRT1,” George Vlasuk, former CEO of Sirtris/GSK told FierceBiotech.
The controversy still hasn’t dissipated, however. As of 2023, scientists like Charles Brenner still don’t believe that resveratrol activates sirtuins. Still, this controversy is beside the point as SirTLab will not be using resveratrol to activate SIRT6. They are doing much more than that.
A Human SIRT6 Activator
“We have developed four different ways to therapeutically target SIRT6 production: messenger RNA (mRNA), small molecules, adeno-associated viruses (AAVs), and antagonists for micro-RNAs that control SIRT6 levels,” says Misholi. “There are different benefits to each approach, and it’s even possible that a treatment could use a combination of two or more. We’ve seen in our experiments that our mRNA therapeutic boosts cellular levels of SIRT6 by 20 times, which is very exciting.”
Many of the technologies mentioned by Misholi have already been tested in humans but with other genes, which gives more feasibility to boosting the SIRT6 gene. And SirTLab has an upper hand when it comes to doing so, as it was co-founded by Dr. Haim Cohen, a world leader in SIRT6 research.
Cohen was trained in Sinclair’s lab at Harvard and has since established his own lab at Bar-llan University in Israel. Cohen and his team were the ones behind prolonging the lifespan of mice by up to 50% by overexpressing SIRT6.

Adding to their brain power, SIRTLab recently announced that Nir Barzilai, M.D., would be joining their team as Chief Medical Advisor and a member of the Board of Directors. Barzilai’s research at Albert Einstein College of Medicine has focused on the genetics of longevity. His team found that a variant of SIRT6 was associated with increased human lifespan, as it was prominent in centenarians, individuals who live 100 years and beyond.
“Nir is among the leaders of the longevity field and biology of aging, with a long and successful record of professional achievements. His involvement with SirTLab, demonstrates the unusual potential of SirTLab approach to longevity, frailty, and other conditions related to old age,” said Misholi to Longevity Technology.
Working Around the FDA’s Poor Definition of Aging
The biggest problem with getting age-reversal therapies approved in the United States is the FDA’s definition of aging. The FDA does not consider aging a disease and drugs are usually approved on the basis that they target a specific disease. However, longevity interventions such as SIRT6 can potentially treat multiple diseases. Alas, the FDA’s rules hinder the progress of would-be longevity medicines whether aging is a disease or not.
To work around the FDA’s traditional procedures, so to speak, SirTLab will test SIRT6 therapies on specific conditions (indications), including fatty liver disease.
“We have many potential indications to consider,” Misholi says. “We have demonstrated that we can treat mice with fatty liver disease, and within three weeks we have reversed it – the liver has become totally healthy. In our mouse studies, we found that increasing the level of SIRT6 in the liver alone had a very strong impact on the activity on the energy level of and the overall health of the mice.”
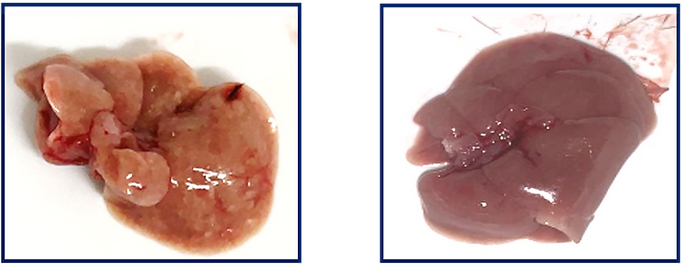
The SirTLab CEO also believes that SIRT6 could alleviate frailty, inflammatory diseases, neurodegeneration, and osteoporosis. Ultimately, treating these conditions could result in extending the human lifespan.
“We strongly believe that the platform we have developed has the potential for delivering the first therapeutic to improve human longevity,” he says. “But, of course, we also need an approved indication from the FDA, so that is why we’ll be going after some specific indications first.”