Highlights:
- Researchers measured brain cell proteins from the blood of patients to identify psychiatric condition markers and therapeutic targets.
- Levels of the NAD+ generating protein NMNAT2 are disrupted in patients with Major Depressive Disorder (MDD).
- Since NAD+ levels also fall as biological age progresses, these findings provide a link between MDD and aging.
Most of us feel blue from time to time, but a persistent dampened mood can indicate a serious health condition. Major depressive disorder (MDD) is a mood disorder characterized by the loss of pleasure, lack of energy, and feelings of inadequacy. Even though MDD is ranked as the top contributor to worldwide disability, we still do not grasp the specific underlying causes, let alone how to treat this psychiatric disorder. However, there are some hints that the root of MDD may involve cellular aging-related defects and dysfunction of mitochondria, the cell’s energy-producing centers. Identifying markers for neuropsychiatric disease-causing mechanisms could be critical for the diagnosis of MDD.
In a study recently published in Molecular Psychiatry, Goetzl and colleagues from the University of California School of Medicine, San Francisco, found that MDD patients have altered levels of proteins involved in aging and mitochondrial function. In particular, there were changes in the levels of proteins involved in the metabolism of nicotinamide adenine dinucleotide (NAD+), an essential coenzyme intimately tied to a myriad of crucial cellular processes that becomes dysregulated with age. The findings suggest that the proteins related to NAD+ metabolism could serve as informative indicators and therapeutic targets of brain health.
Mining blood for MDD markers
Staggering amounts of research have offered up observations on striking mitochondrial alterations in brain cells of patients with major psychiatric diseases. These findings suggest that mitochondrial abnormalities may represent fundamental disease-causing mechanisms in MDD.
By looking at the blood of MDD patients, researchers have begun to find compounds that increase our understanding of how this psychiatric disorder develops and unfolds. However, despite the quantitatively striking changes in the concentrations of some blood-based biomarkers in MDD, these alterations often have lacked disease specificity and predictive capability regarding changes in severity or responsiveness to the treatment of MDD.
One recently emerging technology relies on analyses of blood mRNAs – the molecules that are produced as an output of gene activity and act as intermediates during protein production. By tracking mRNAs in blood, researchers aim to predict the course and severity of depression and mania, as well as to identify potentially beneficial drug treatments.
MDD is associated with abnormal levels of NAD+ metabolism proteins
To find reliable markers and targets for MDD treatment, Goetzl and colleagues previously developed a platform that analyzes vesicles – tiny fluid-filled sacs – derived from brain neurons located in the blood. Recent analysis has shown that the proteins inside these brain-derived vesicles are representative of the proteins in brain cells, such as mitochondrial components.
The researchers’ past analysis compared proteins inside brain-derived vesicles from subjects with first episode psychosis to subjects without MDD to reveal abnormalities in the levels of mitochondrial proteins and some other brain-specific proteins. In this study, Goetzl and colleagues looked at how the levels of mitochondrial proteins and other markers changed before and after treatment with a common antidepressant medication called a selective serotonin reuptake inhibitor (SSRI) in participants either with or without MDD.
Compared to participants without MDD, many of the mitochondrial proteins studied were decreased in all (responder and non-responder) MDD patients before SSRI treatment. These included proteins involved in the maintenance and survival of mitochondria and the mitochondrial generation of energy.
In addition, NMNAT2 (nicotinamide mononucleotide adenylyltransferase 2), the enzyme involved in the synthesis of NAD+, was also decreased. The only protein studied related to NAD+ metabolism that was increased in these blood samples derived from MDD patients was SARM1. This enzyme consumes NAD+ and is well known for its pro-neurodegenerative function. Goetzl and colleagues suggested that the differences observed in the levels of NMNAT2 and SARM1 would lead to lower mitochondrial concentrations of NAD+.
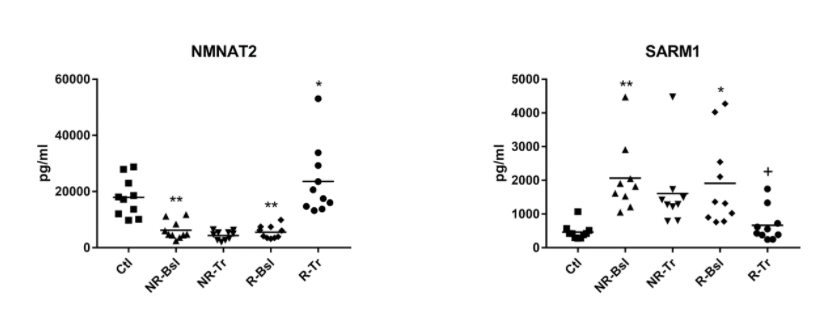
The work of Goetzl and colleagues reinforces the importance of mitochondrial function in MDD and reveals the potential applicability of using NAD+ signaling as a therapeutic target for MDD. Although the functional consequences of the changes in protein concentrations were not studied in MDD patients, NAD+ levels may be reduced. These results suggest that the pathology of MDD is similar to brain aging, as NAD+ depletion and loss of mitochondrial function have already been implicated in the aging process.
How Does NAD+ Level Maintenance Relate to Brain Health?
Many hallmarks of brain aging are related to NAD+ depletion. The decline in NAD+ that occurs during the aging process contributes to age-dependent decreases in mitochondrial function. Aging is also associated with the accumulation of reactive oxygen species (ROS), which cause damage to mitochondria. NAD+ plays a crucial role in combating these ROS and the mitochondrial damage they cause. Excessive ROS is also associated with neuropsychiatric disorders like MDD. Another hallmark of brain aging that also occurs in MDD is inflammation, and NAD+ suppresses inflammation by mediating the removal of damaged mitochondria.
Also, since this study shows increased levels of SARM1 in MDD patient blood, targeting this enzyme that consumes NAD+ may have a therapeutic effect on this neuropsychiatric condition. This suggestion is in line with a proposal by Hopkins and colleagues suggesting that a drug targeting SARM1 could be developed to slow the progression of brain aging as well as neurodegeneration.
Overall, these findings help clarify the relationship between MDD and brain aging, which seems to be linked together by proper NAD+ signaling and mitochondrial function, or lack thereof. In light of the results from Goetzl and colleagues, implicating similar dysfunction in MDD, the link between MDD and aging becomes stronger. Advances in understanding how NAD+ and mitochondria support healthy brain aging may lead to novel approaches for treating a range of neurological disorders, including MDD.