As the global population continues to age and grow in number, age-related cognitive impairment has progressively become a pressing issue. A deleterious neurological mechanism called the integrated stress response (ISR) becomes apparent as humans age and promotes age-associated cognitive impairment.
In a study published on December 1st (year) in the open-access journal eLife, Rosi and colleagues from UC San Francisco found that providing old-aged mice with an ISR inhibiting drug resulted in restored cognitive abilities normally seen at youth. In addition, the integrated stress response inhibitor (ISRIB) induced neuron repairment, which likely contributed to the observed boosts in brain function.
“ISRIB’s extremely rapid effects show for the first time that a significant component of age-related cognitive losses may be caused by some kind of reversible physiological ‘blockage’ rather than more permanent degradation,” stated Susana Rosi, Ph.D., lead author of this study and a professor in the Neurological Surgery and Physical Therapy and Rehabilitation Science Departments at UC San Francisco, in a press release.
Age-Induced ISR Activation Impairs Neuron Function
The ISR normally pinpoints protein production abnormalities in cells caused by cancer-promoting gene mutations, viral infections, or misfolded proteins that accumulate with age. To address these problems, the cell halts protein production. This method can remove defective cells, but when cells lose their ability to operate normally, promoting activation of this mechanism in a tissue like the brain can initiate age-related cognitive impairment.
The Experimental Drug ISRIB Resets the Age-Induced Brain Stress Response
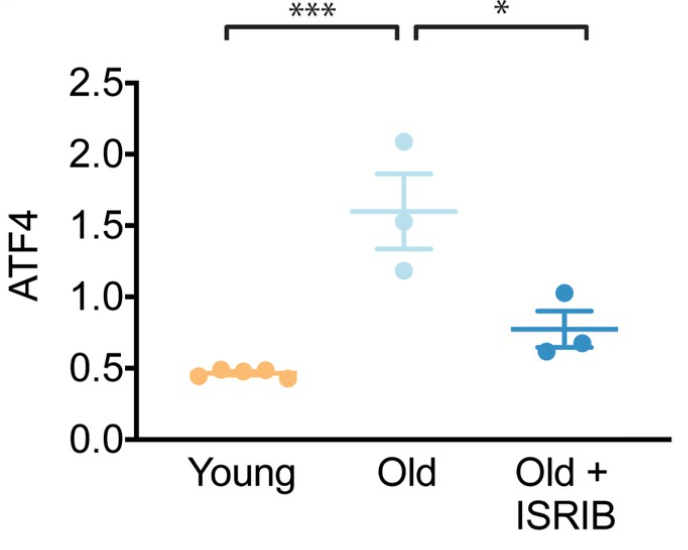
When investigators administered ISRIB to aged mice, the ISR in the subjects’ brains was reset. These results were seen upon examination of the effects presented 18 days after aged mice were injected with ISRIB daily for three successive days. Normally, ISR induces elevation of the protein ATF4, but injecting aged mice with the experimental compound ISRIB reversed the age-associated increase in ATF4 protein levels. Furthermore, investigators noticed a sustained decrease in ATF4 protein levels after evaluating the results 18 days after aged mice were injected with ISRIB.
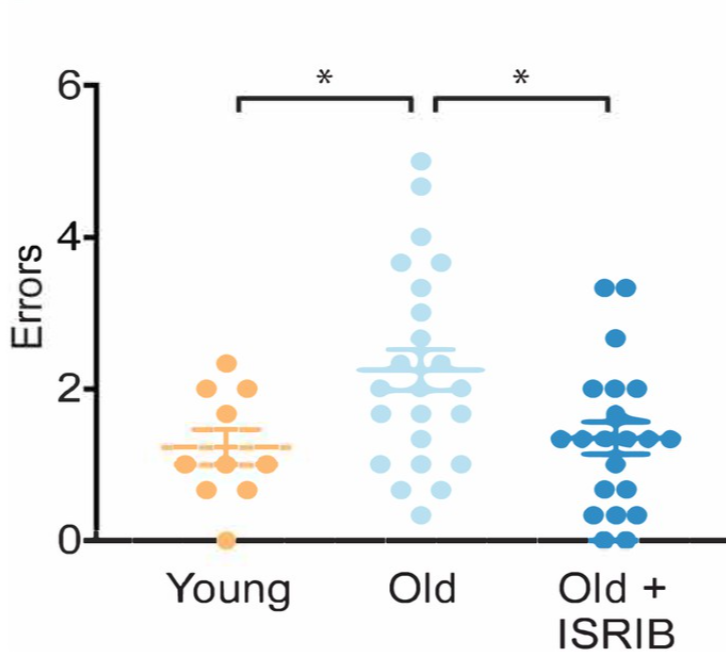
ISRIB injections also restored age-induced spatial learning and memory deficiencies in mice. Investigators compared the performance of young and old mice in a water maze to evaluate how ISRIB alters age-associated cognitive impairments. In the special memory test, mice are repeatedly and randomly stationed into an opaque water pool and are responsible for locating a concealed platform. The mice are allowed to utilize spatial cues contained within the pool to locate the platform. In the study, mice were given two days to figure out how to utilize the spatial cues to find the platform concealed under opaque water before undergoing testing. Results showed that young mice made one error on average before successfully finding the escape platform. On the other hand, older mice made three errors on average before locating the platform. Once aged mice were given ISRIB, they made two errors on average before finding the escape platform, thus suggesting that administration of ISRIB improved their memory of the platform location. Notably, these memory enhancements continued for weeks after mice were administered ISRIB.
ISRIB Reversed Age-Related Changes to Neurons
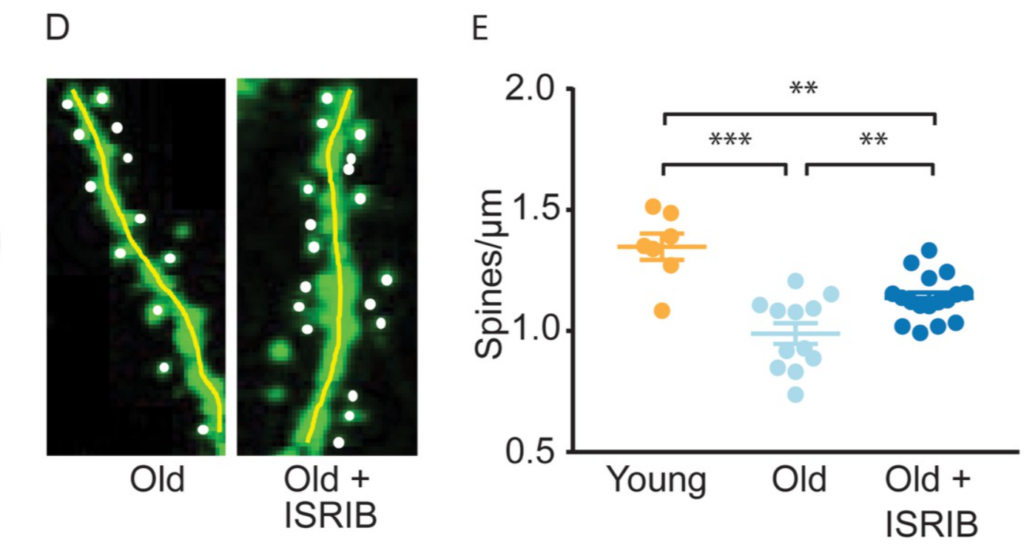
Further observations showed that treating aged mice with ISRIB reversed age-associated alterations in neurons located in the hippocampus, a region of the brain responsible for memory and learning. The lack of changes in these neurons likely explains the improved memory seen in aged mice. In addition, investigators observed enhanced neuron excitability after injecting aged mice with a single dose of ISRIB one day prior to examining neuron electrical activity. Furthermore, aged mice treated with ISRIB exhibited increased density in cellular structures called dendritic spines, which are associated with communication between neurons.
The investigators of this study postulate that activation of ISR and subsequent blocking of cellular protein production may be a key contributor to various neurological disorders. Thus, researchers believe that conditions like Alzheimer’s disease, traumatic brain injury, and frontotemporal dementia could potentially be treated with ISRIB.
Future Directions for Utilizing ISRIB
“The data suggest that the aged brain has not permanently lost essential cognitive capacities, as was commonly assumed, but rather that these cognitive resources are still there but have been somehow blocked, trapped by a vicious cycle of cellular stress,” said Peter Walter, Ph.D., another author of the study and professor in the UC San Francisco Department of Biochemistry and Biophysics in the press release. “Our work with ISRIB demonstrates a way to break that cycle and restore cognitive abilities that had become walled off over time.”
Calico, a company stationed in South San Francisco that conducts research on aging biology, received the license to ISRIB. According to Peter Walter, Ph.D., various pharmaceutical companies are also attempting to treat certain diseases by targeting ISR. “It almost seems too good to be true, but with ISRIB we seem to have hit a sweet spot for manipulating the ISR with an ideal therapeutic window,” said Walter in the press release.