Highlights
- Protein clumps called amyloid plaques accumulate during muscle aging and disease across species.
- Late-life NAD+ boosting reduces amyloid plaques during aging to improve healthspan and mitochondrial function.
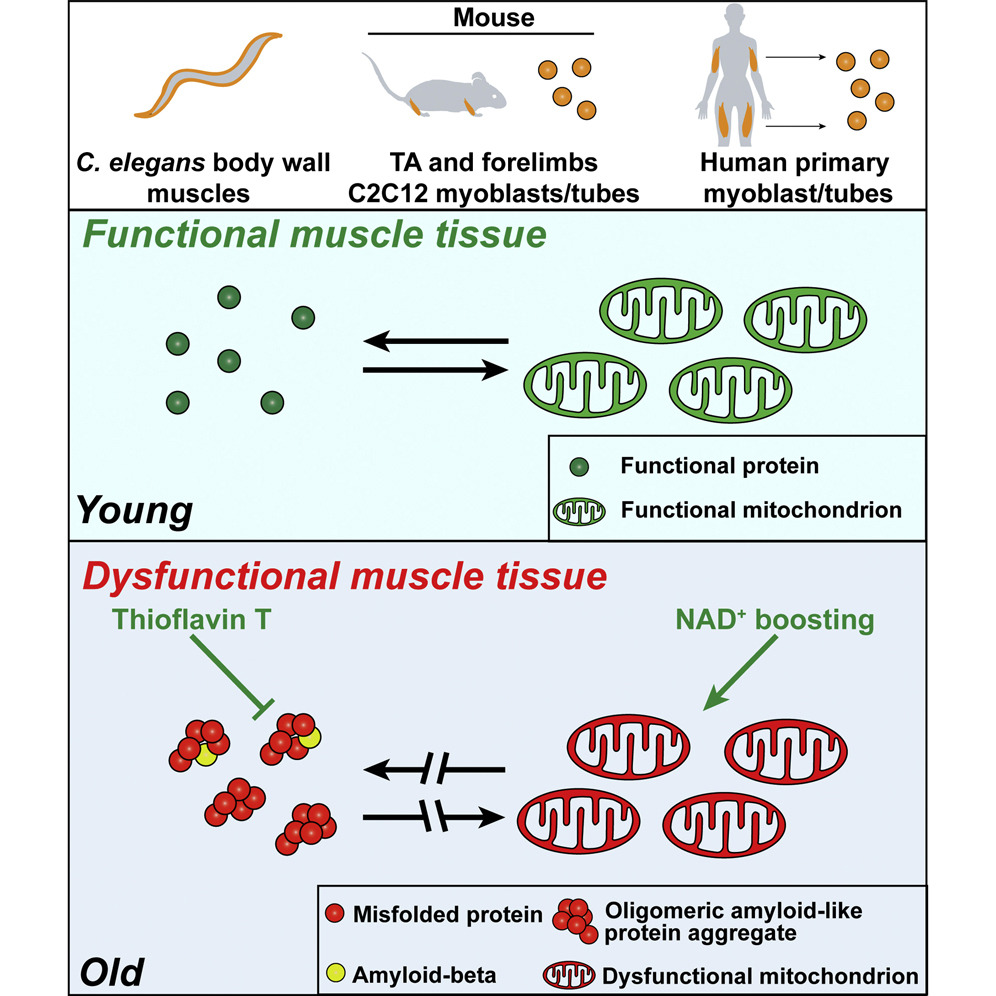
Aging is characterized by the imbalance of proteins — disrupted proteostasis — and dysfunctional mitochondria. These characteristics are often seen in age-related degenerative disorders with features like detrimental protein aggregation in Alzheimer’s disease and inclusion body myositis (IBM), a progressive muscle disorder. However, how proteostasis falls in aged muscle is not fully understood, and it is not clear whether harmful protein conglomerations called amyloid deposition, a hallmark of Alzheimer’s disease and IBM, also plays a role in the aging muscle.
Romani and colleagues from the Ecole Polytechnique Fédérale de Lausanne in Switzerland published an article in Cell Reports showing that, during natural aging across species, muscle tissues accumulate amyloid-like deposits. The researchers also discovered the buildup of these deposits are reversible, which can be diminished by interventions geared toward restoring a mitochondrial functional balance, such as by increasing nicotinamide adenine dinucleotide (NAD+) metabolism, even at the beginning of aging processes.
Importantly, they show that reducing the accumulation of amyloid-like deposits during aging with NAD+ boosters suffices to improve mitochondrial function in muscle and beneficially impacts health and lifespan. “Drugs that boost mitochondrial quality control could therefore be tested in the clinic to reverse these age-related proteotoxic aggregates and rejuvenate tissues,” says Mario Romani, the lead author of the study, in a press release.
Amyloid aggregate accumulation in aging muscle cells across species
Romani and colleagues found that the activity of gene signatures linked with protein balance and accumulation along with mitochondria are altered in aging muscle and in diseases. They looked at data from human muscle datasets of aging, muscle diseases linked to genetics like muscle dystrophies, and muscle protein pathologies, such as IBM and amyotrophic lateral sclerosis (also known as Lou Gherig’s disease). Interestingly, amyloid aggregation pathologies and protein-misfolding-related gene sets were among the most induced in all the aging muscle and disease conditions analyzed.
Moreover, Romani and colleagues show aggregations of amyloid-like deposits and dysfunctional mitochondria during natural aging in the body wall muscle of roundworms called nematodes, human primary myotubes (mature muscle cells), and mouse skeletal muscle, partially modeling IBM. Also, they have demonstrated a critical role of balanced NAD+ levels to control amyloid conglomeration levels.
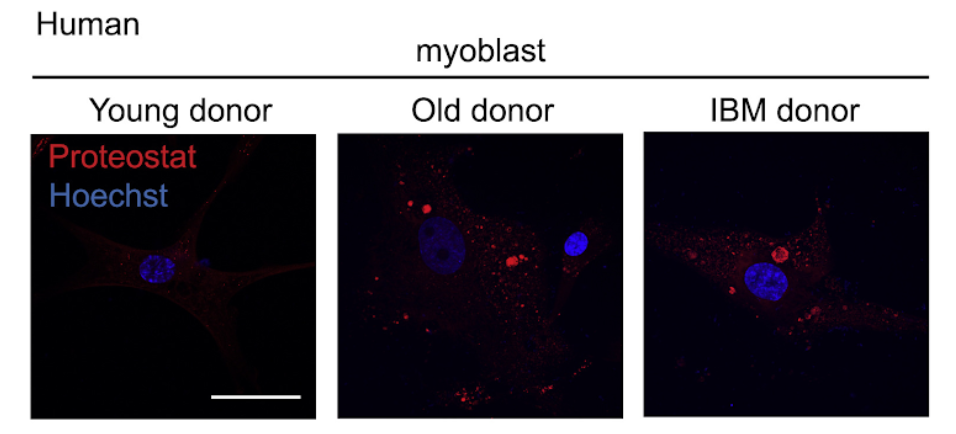
These analyses suggest that there is an overall declining mitochondrial function paired with disturbances in cellular proteostasis of skeletal muscle in established proteinopathies, such as IBM, in aging, as well as in other long term muscle conditions, including muscular dystrophies. These shared disturbances, suggestive of increased protein misfolding and amyloid pathology hence may represent a major hallmark of muscle aging and disease. “These abnormal proteotoxic aggregates could serve as novel biomarkers for the aging process, beyond the brain and muscle,” says Auwerx.
NAD+ boosting reduces amyloid formation and improves mitochondria during aging in worms and mammals
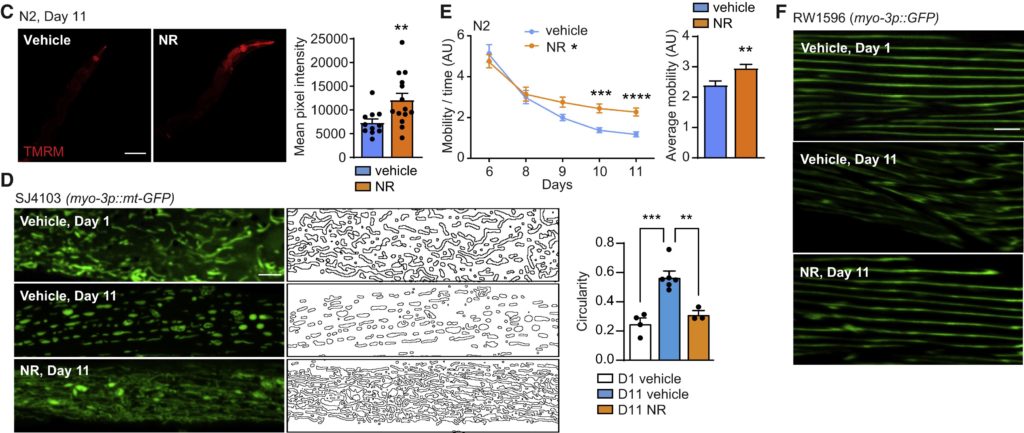
Romani and colleagues also showed that the evolutionarily processes of amyloid pathology and aging-associated loss of protein balance are directly linked to NAD+ levels — a vital molecule with essential roles in regulating cell energy, function, and health that diminishes with age. Since NAD+ balance is crucial to control age-related muscle amyloidosis, Romani and colleagues treated roundworms modeling amyloid-beta muscle protein toxicity, human aged myotubes, and old mice with the NAD+ boosters nicotinamide riboside (NR) and olaparib (AZD). They then tested for altered mitochondrial function, muscle protein balance, and amyloid plaque accumulation.
Late-life treatment with either NR (1 mM) or olaparib (AZD; 300 nM) had a substantial impact on protein balance in aged worms, with amyloid-like deposits being reduced by both molecules. Late-life NAD+ boosting interventions also improved mitochondrial structure and membrane potential — an indicator of membrane function — in aged animals and increased mitochondrial DNA content, indicative of greater mitochondrial proliferation (biogenesis). This furthermore improved organism fitness, measured by spontaneous movement, muscle integrity, and percentage of paralyzed worms and those that died at day 18.
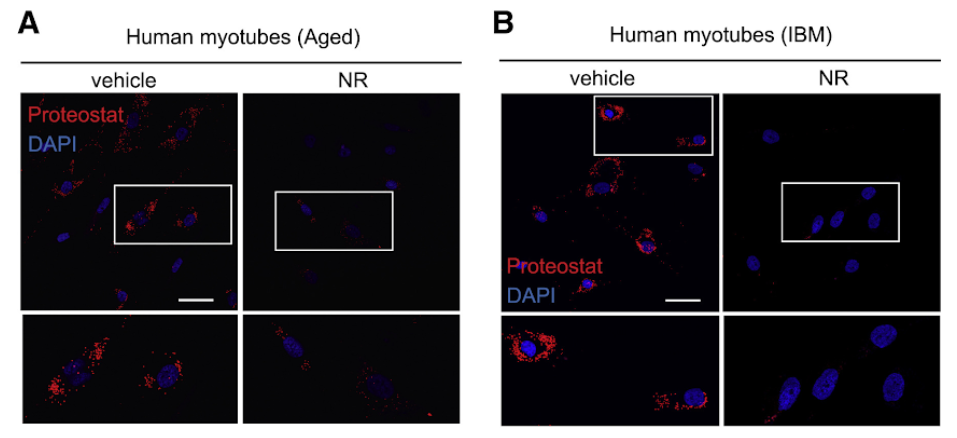
To translate the worm findings to mammals, they also treated human myotubes obtained from aged individuals and IBM patients with NR and AZD and examined the presence of amyloid-like proteins. Importantly, both treatments diminished the number of cell protein aggregates derived from aged or IBM donors and mouse myotubes. Interestingly, co-treating cells with a drug called a global integrative stress response (ISR) inhibitor (ISRIB), attenuated the decline in aggregates seen with NAD+ boosting. The reduced amyloid-like deposits went along with an improvement of the mitochondrial membrane potential, increased mitochondrial energy production, and more fine mitochondrial formations.
The future of restoring age-related muscle deterioration with NAD+
“Our results in [worms], mice, and humans support the notion that natural aging is typified by muscle amyloidosis and, very importantly, that late-life treatments aimed at restoring metabolic and mitochondrial homeostasis also increase cellular and organismal proteostasis, therefore beneficially impacting on health- and lifespan,” the authors concluded. What’s more, NAD+ boosting improves protein balance and attenuates amyloid deposit formation across multiple species, including humans.
The study’s promising outcomes could trigger clinical trials to test the effectiveness of NAD+ therapeutics in treating human diseases related to amyloidosis. Future work may also focus on finding the ideal NAD+ boosting method by testing different precursors besides NR, like nicotinamide mononucleotide (NMN).