Key Points
- USC researchers pinpoint 15 loci – specific positions of a gene or DNA sequence on a chromosome – that accelerate or halt brain aging.
- Genetic analysis reveals an overlap between genes associated with schizophrenia, depression, insomnia, and cognitive functioning.
Our genes, along with environmental factors, dictate how our brains change with age . However, scientists are still exploring the timeline of these changes and which genes are responsible for speeding up or slowing down brain aging.
Published in the journal Nature Neuroscience, researchers from the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) consortium analyzed brain scans from 15,640 subjects (aged 4 to 99) to examine the rate of tissue growth and atrophy in various brain regions, particularly those linked to memory, cognition, and behavior. The researchers also pinpointed 15 genetic loci that either accelerate or halt brain aging. What’s more, investigators highlighted an overlap between genes associated with schizophrenia, depression, insomnia, and cognitive functioning. Taken together, the findings from the study provide insight into potential new drug targets that improve overall brain health and combat neurodegenerative disorders like Alzheimer’s.
Key Structural Brain Changes Across Lifespan
Prior to genetic analysis, Thompson and colleagues measured the rate of change of 15 brain structures across a lifespan. 14 of these brain structures, particularly regions responsible for learning and memory (hippocampus), exhibited notable structural decline over time. While the structure of the hippocampus appeared to remain stable between the ages of 7 and 35, imaging revealed structural reductions occurring between the ages of 55 and 75. This hippocampal shrinkage is known to hamper cognition and interfere with memory formation, both of which are hallmark symptoms of Alzheimer’s disease.
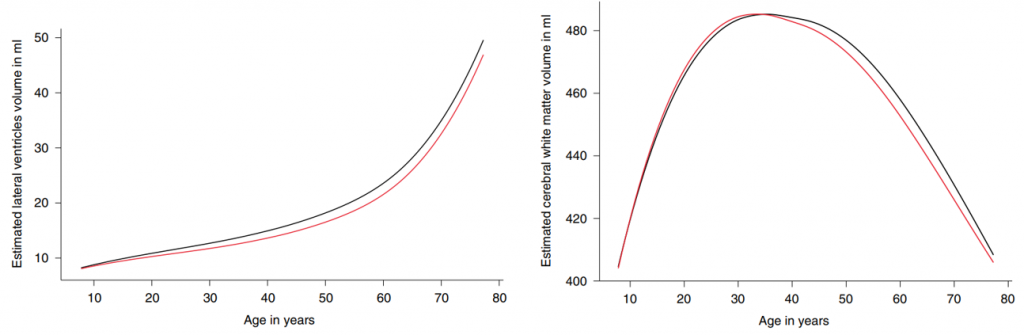
Interestingly, only one brain structure, the lateral ventricles, seemed to progressively increase with age. Lateral ventricles are filled with cerebrospinal fluid (CSF), a clear liquid that delivers structural support to the brain and assists with nutrient circulation and waste removal. Lateral ventricle enlargement is heavily seen in patients with Alzheimer’s dementia, which makes sense given that the surrounding brain tissue shrinks and becomes susceptible to permanent damage following increased lateral ventricle volume.
So, while these initial findings provide insight into common structural brain changes during aging, Thompson and colleagues were more interested in pinpointing genetic loci that could accelerate or halt brain aging.
“The big game-changer here is discovering locations on the chromosome that speed up or slow down brain aging in worldwide populations. These can quickly become new drug targets,” states Paul Thompson, USC ENIGMA researcher and lead author of the study.
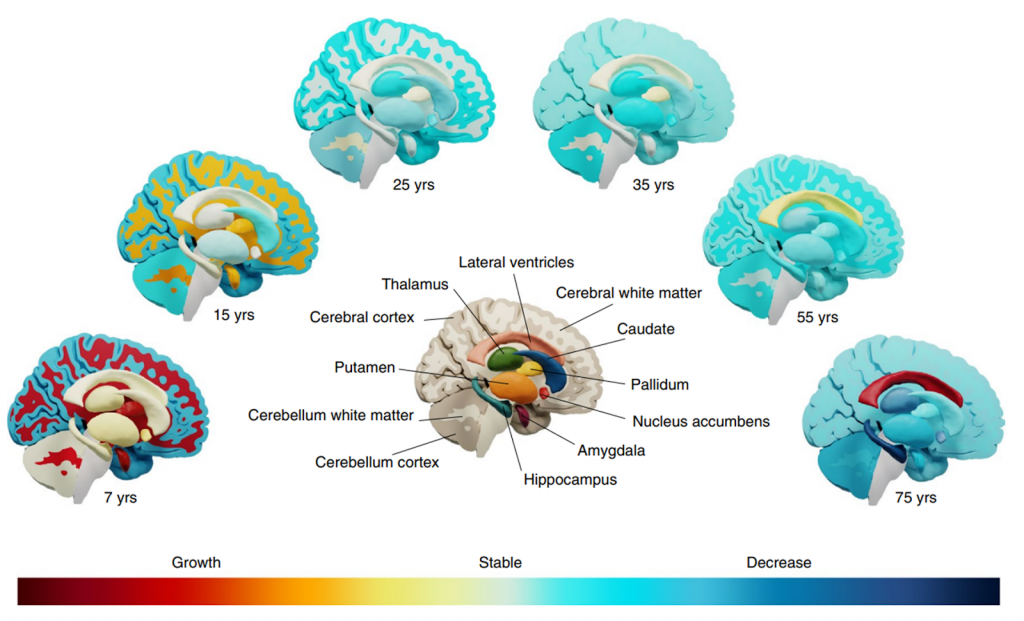
Genes Controlling Rates of Brain Change
Genetic analysis successfully located 15 genomic loci that boosted or slowed down brain aging. The investigators categorized each genetic variant — DNA sequence within a gene that varies between individuals — within each loci as either age-dependent or age-independent. Here, age-dependent gene variants are those that change during brain development and into adulthood, whereas age-independent gene variants remain constant over a lifetime. The most significant age-independent genetic variant was located in the GPR139 gene, which encodes receptor proteins responsible for facilitating responses to hormones and other extracellular – outside the cell – signals. Additionally, investigators found a strong association between GPR139 and rate of change in lateral ventricle volume, highlighting a potential new target to treat an abnormality – increased lateral ventricle volume – linked to Alzheimer’s.
Among the loci that contained statistically significant age-dependent genetic variants, two that stood out were located in the following two genes: APOE (apolipoprotein E) and DACH1. APOE is another famous Alzheimer’s linked protein that, in the absence of cholesterol, drives the degeneration of synapses – neuronal junctions that allow electrical or chemical signals to be transmitted between neurons. Moreover, investigators found a strong association between the genetic variant in APOE and the hippocampus’ rate of structural change, displaying gradual growth into adulthood and subsequent shrinkage.
Similarly, the genetic variant seen in DACH1, which participates in regulating gene activity, also promotes accelerated growth during childhood and gradual decline upon aging. DACH1 is asscosiated with changes in cerebral white matter, the connections in our brain that modulate communication between multiple brain regions to be integrated for higher brain functions. Overall, these findings show that there are specific gene variants associated with structural changes to the aging brain.
Identifying Novel Drug Targets
By tying specific loci to rates of structural brain changes, Thompson and colleagues have successfully widened our scope of understanding on brain aging and open the door for treatments that can optimally target biological pathways involved in overall brain health. Additionally, they even confirmed a genomic overlap between genes associated with schizophrenia, depression, insomnia, and cognitive functioning. This suggests that there might be a shared mechanism that can be targeted to treat multiple neurological disorders at once. Although the study’s findings helped clarify brain-structure-specific associations, more longitudinal brain studies are needed to solidify the connection between age and rates of change in brain structures across one’s lifespan.