Key Points:
- Of the thousands of neurons that connect to the heart, some gradually disconnect with aging, and this occurs in parallel to senescent cell accumulation.
- Senolytics reduce senescent cells in the heart and prevent neurons from disconnecting from the heart.
- Senolytics also prevent irregular heart rhythms called arrhythmia, which has previously been linked to aging and neuron connection losses.
Our brain is connected to our heart by thousands of neurons. However, conditions of cardiac aging, such as irregular heart rhythms, can be triggered by neurons disconnecting from the heart. Now, scientists from Goethe University Frankfurt in Germany have found that senescent cells may promote this disconnection.
Wagner and colleagues report in Science that aging gradually reduces the number of axons — projections emanating from neuron bodies — that connect to the heart in old mice. Furthermore, they show that this reduction can be prevented by senolytics. In turn, senolytics are shown to prevent irregular heart rhythms, which are associated with altered neuron connections and aging. The authors conclude,
“These data suggest that senescence-mediated regulation of [axon] density contributes to age-associated cardiac dysfunction.”
Senolytics Restore Heart-Brain Connection and Counteract Age-Related Cardiac Conditions
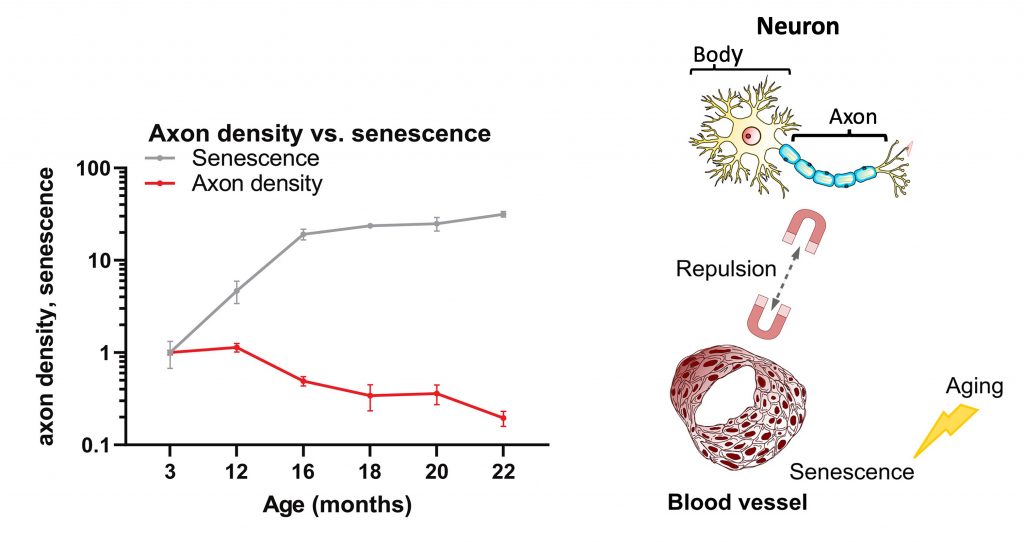
To explore how the heart-brain connection changes with age, Wagner and colleagues measured the density of axons within the hearts of aging mice. The results showed that axon density started to decline when mice were 16 months old, equivalent to about 52 human years. It was also shown that this decline paralleled an increase in senescent cells — dysfunctional cells that accumulate with aging.
Further experiments revealed how an increase in senescent cells could trigger a reduction in axon density. Usually, blood vessel cells secrete molecules that guide neurons to blood vessels. However, it was found that senescent blood vessel cells secrete molecules that repel neurons aways from blood vessels. In this way, senescent cells could cause some neurons to become disconnected from the heart’s blood vessels.
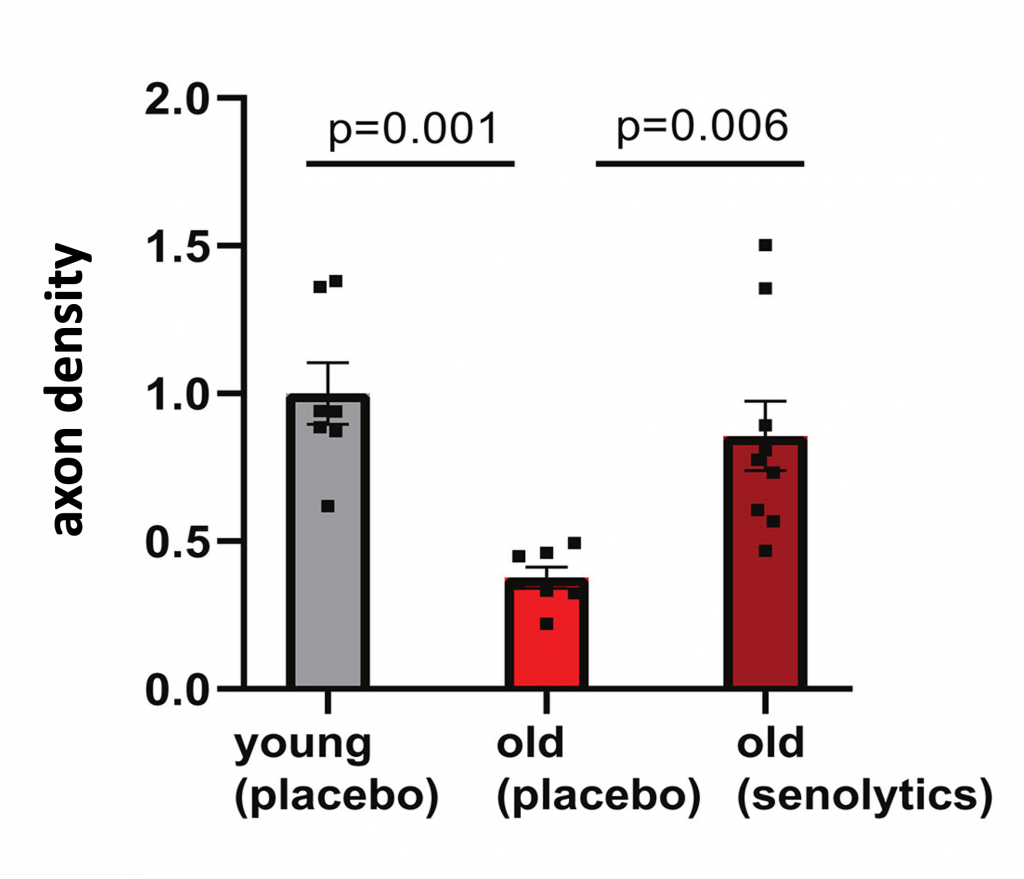
Since senolytics selectively eliminate senescent cells, Wagner and colleagues tested whether 5 mg/kg of dasatinib — a synthetic senolytic — combined with 50 mg/kg of quercetin — a natural senolytic found in plants — could prevent axons from disconnecting from the heart. To do so, the senolytics were fed to old mice (18 months, equivalent to about 56 years in humans) for 3 consecutive days every other week for 2 months.
As a result of senolytic treatment, axon density in old mice reached levels similar to young mice. Furthermore, old mice given a placebo had about half the axons of both young mice and senolytic-treated old mice. These findings suggest that senolytics can prevent the disconnection of neurons from the heart that occurs with aging.
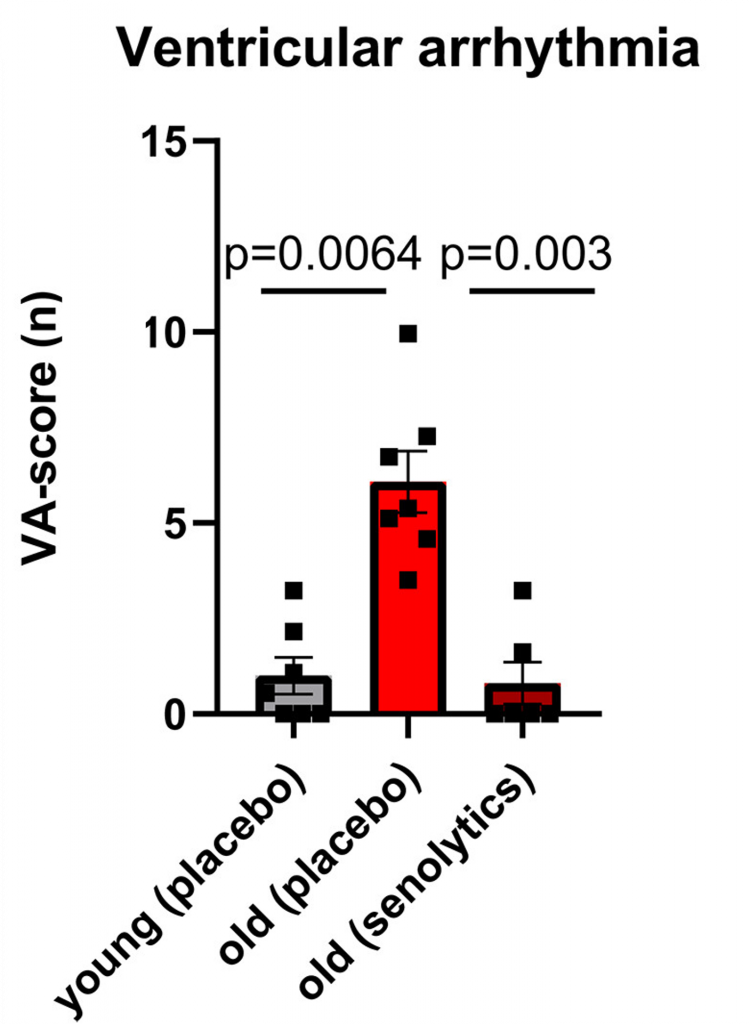
Our brain and heart communicate via electrical signals sent over long distances through axons. With age, we become more vulnerable to irregular heart rhythms called ventricular arrhythmia (VA), which are associated with altered axon connections. Wagner and colleagues found that much like old humans, old mice are more susceptible to VA. However, treating old mice with senolytics greatly reduced their VA susceptibility. Indeed, senolytic-treated old mice were just as susceptible to VA as young mice, suggesting a reversal of this condition of cardiac aging.
Can Senolytics Counteract Heart, Muscle, Brain, and Bone Aging?
Overall, the findings Wagner and colleagues suggest that senolytics (dasatinib and quercetin) are capable of reversing heart aging by preventing neurons from disconnecting from the heart. This same senolytic combo has been shown to enhance muscle regeneration, promote small intestine regeneration, preserve memory and mitigate neurodegeneration, and ameliorate bone loss in recent animal studies. Therefore, as longevity expert Dr. Eric Verdin has alluded to, we may see dasatinib and quercetin as an anti-aging agent for humans in the near future.