Key Points:
- High-intensity interval exercise (HIIE) but not steady state exercise (SSE) reduces p16 mRNA in the muscle of sedentary men.
- SSE but not HIIE reduces DNA damage in the muscle of sedentary men.
Most of our cells have short lifespans, dividing a few hundred times before entering replicative senescence — when a cell no longer divides. Not only do senescent cells not divide, but they can also cause organ damage if not removed. For this reason, the accumulation of senescent cells is thought to play a major role in driving the aging process. While senolytics — compounds that remove senescent cells — hold promise in deterring the aging process by mitigating organ deterioration, a new study demonstrates that exercise may have similar effects, but only if at sufficient intensity.
Researchers from the University of Taipei in Taiwan report in Aging that the senolytic effect of exercise on muscle is intensity-dependent.
“The intensity effects of aerobic exercise on cellular senescence and DNA repair response of human skeletal muscle have not been previously documented,” say the authors.
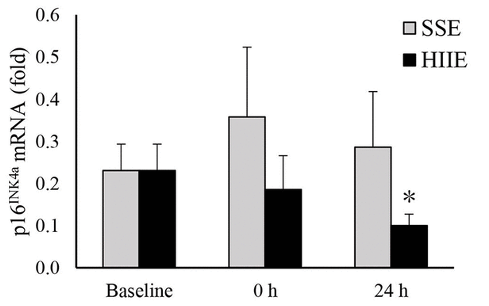
Jean and colleagues show that the senescent cell gene marker p16 is reduced one day after HIIE. However, p16 is not reduced one-day following low-intensity SSE. In contrast, DNA damage is reduced 24 hours following SSE, but not HIIE. These findings demonstrate the potential complementary benefits of SSE and HIIE.
Exercise Leads to Reduced Senescence and DNA Damage
Jean and colleagues hypothesized that sufficient exercise intensity is necessary for exercise to have a senolytic — senescent cell-reducing — effect. This was based on previous studies showing that resistance training but not low-intensity aerobic exercise reduces senescent markers. Therefore, the researchers had sedentary men (approximately 26 years of age) participate in SSE and HIIE.
Peak volume oxygen consumption (VO2peak), derived from the rate of oxygen consumed during maximal physical exertion, was measured from all participants one week before SSE and HIIE. Exercise intensity was based on VO2peak, whereby SSE was assessed by 10 minutes of cycling at 60% VO2peak and HIIE by 20-second burst of cycling at 120% VO2peak with 20 seconds of rest in between up to 15 times in a row. Samples from the vastus lateralis thigh muscle were taken 3 weeks before, directly after, and 24 hours after exercise.
One of the genes elevated in senescent cells is called p16, which promotes replicative senescence. Jean and colleagues found that 24 hours after HIIE, p16 mRNA levels in the muscle of sedentary men were cut in half. In contrast, there were no significant reductions in p16 mRNA 24 hours after HIIE. These results support the hypothesis that sufficient exercise intensity is necessary to reduce senescent cell burden.
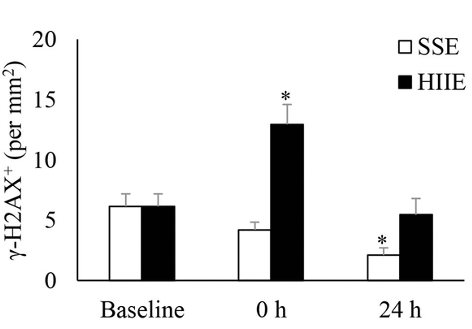
DNA damage is associated with aging and seems to be a natural consequence of cellular divisions throughout a lifespan. To assess DNA damage, Jean and colleagues measured a standard marker for DNA repair called γ-H2AX. They found that γ-H2AX increased immediately after HIIE, whereas it did not significantly change immediately after SSE. Furthermore, γ-H2AX did not significantly change 24 hours after HIIE, whereas γ-H2AX was reduced by half 24 hours after SSE. Since DNA damage is associated with senescence, these results suggest that HIIE leads to the efficient clearance of senescent cells. The results also suggest that SSE leads to a reduction in DNA damage.
Is the Senolytic Effect How Exercise Slows Muscle Aging?
As we age, our muscles lose mass and strength. It was previously shown that 4 weeks of high-intensity interval training (HIIT) increased muscle gains in older individuals. In contrast, 12 weeks of treadmill running for 45 minutes does not increase the lean mass of older individuals. Our muscles cannot grow in response to exercise unless our muscle stem cells are functioning properly. It is thought that with age, our muscle stem cells enter a senescent state, blocking muscle growth. If senescent stem cells are reduced as a result of high-, but not low-intensity exercise, this may, in part, explain why only older individuals who participate in high-intensity exercise see muscle gains.
Considering that other studies show senolytics could potentially ameliorate muscle aging, it seems possible that high-intensity exercise could do the same. Exercise has also been associated with reducing senescent cells in the colon of older adults. Therefore, the anti-aging effects of exercise could, at least in part, be due to the reduction in senescent cells. More studies will be needed to determine the long-term effects of different exercise regimens on senescent cells and how they relate to aging.