Key Points:
- The synthetic glucocorticoid dexamethasone (Dex) — used to simulate psychological stress by activating the stress hormone cortisol — increases cell death in cells donated by humans.
- Dex reduces cell division rates, indicating premature cellular senescence — associated with driving the aging process.
- Three different biological age calculators show that Dex accelerates aging.
Our psychological stress is associated with elevated levels of the stress hormone cortisol and an up to 67% increase in whole-body energy expenditure. In response to stress, our cells increase survival processes to promote resilience, termed allostasis. However, chronic activation of allostasis — allostatic overload — could contribute to cellular aging. Now, a new study from Columbia University shows that such an allostatic load is associated with hypermetabolism (increased energy expenditure), providing the cellular basis for how psychological stress could contribute to aging.
“Our… findings highlight hypermetabolism as a core manifestation of cellular allostatic load, providing to our knowledge the first quantitative estimate of the energetic cost of allostatic load at the cellular level, increasing energy expenditure by >60%,” say Bobba-Alves and colleagues, the authors of the study.
The Columbia University researchers, in collaboration with scientists from other institutions like UCLA and Yale, report in a preprint posted on BioRxiv that chronic stress increases energy expenditure and accelerates aging. In fibroblast cells from three human donors, Bobba-Alves and colleagues show that cell death is increased by inducing stress with Dex. Dex also promotes cellular features of aging, including replicative senescence and telomere shortening. Furthermore, Dex accelerates biological aging, as measured by three different aging clocks.
“Our results demonstrate that glucocorticoid signaling triggers energy dependent allostatic recalibrations, which translates into age-related signs of allostatic overload,” say the authors.
Stress Induction Triggers Cell Death and Accelerates Cellular Aging
Human fibroblasts were isolated from healthy donors and exposed to the glucocorticoid activator Dex. This triggered allostatic effects such as a 33% reduction in cell size and a 3.3-fold increase in cell death. Since cell growth and survival are dependent on cellular energy — ATP, the researchers measured ATP production. They found that Dex increased ATP production from mitochondria by up to 4-fold. Furthermore, cell death was correlated with this increased energy demand.
The authors conclude, “stress induced hypermetabolism was tightly linked to time of cell death, similar to the associations with mortality observed in humans.”
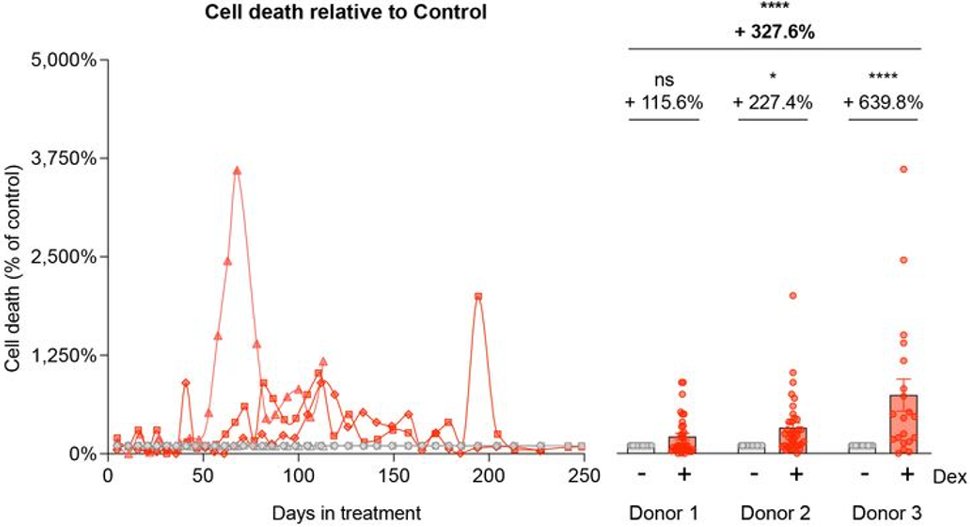
Most of our cells have a limited lifespan, meaning they can only replicate a few hundred times before becoming senescent cells. Senescent cells are associated with driving the aging process.
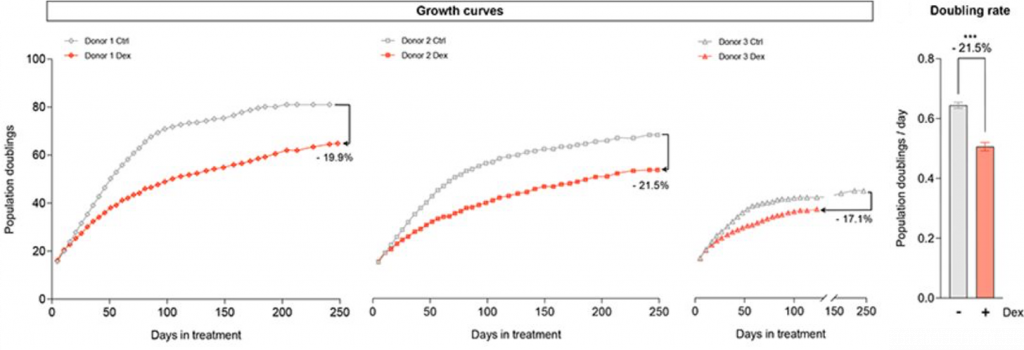
To assess replicative senescence (when cells stop dividing), Bobba-Alves and colleagues measured the maximal number of population doublings (cell divisions) accomplished by donor cells exposed to Dex. They found that this doubling rate was reduced by about 21%, suggesting premature senescence.
As our cells replicate, the repeated sequences of DNA at the end of our chromosomes called telomeres shorten. This telomere shortening has long been associated with the aging process, especially replicative senescence. To examine the basis for Dex-triggered reduced cellular lifespan, Bobba-Alves and colleagues measured telomere length. It was found that Dex increased the rate of telomere shortening, suggesting accelerated aging.
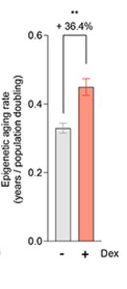
Epigenetic age — a biological age estimation based on molecular tags that turn genes on and off — is highly correlated with chronological age. The researchers found that Dex accelerated the epigenetic age of donor cells with three (out of five) different epigenetic clock calculations. These findings further reveal how stress could accelerate aging (i.e. reprogramming gene activation patterns).
“Our longitudinal data using well-established aging markers validated in humans such as… telomere length, and epigenetic clocks, also link hypermetabolism to accelerated aging biology, as features of cellular allostatic overload.”
The Effects of Stress are Mimicked by Patients with a Disease that Shortens Lifespan
In another study led by Dr. Martin Picard, it was found that cells from patients with a rare mitochondrial disease also exhibit hypermetabolism and shortened cellular lifespan similar to cells exposed to hormonal stress.
“The findings were made in cells from patients with rare mitochondrial diseases, yet they may also have relevance for other conditions that affect mitochondria, including neurodegenerative diseases, inflammatory conditions, and infections,” says Dr. Picard.
Since patients with this mitochondrial disease die prematurely, it would seem that cellular hypermetabolism and human lifespan are linked, meaning that physiological stress could shorten our lifespan by triggering hypermetabolism.
“Hypermetabolism may be a key reason why most cells deteriorate as we get older,” Picard adds.
Can the Effects of Stress on Cellular Aging Be Countered by Boosting NAD+?
In the same type of cells (human fibroblast) used by Bobba-Alves and colleagues, another research group from Korea found that nicotinamide increased cellular lifespan by over 1.5-fold. Nicotinamide is an NAD+ precursor known to boost NAD+ levels. The Korean researchers, Kang and colleagues found that nicotinamide lowered mitochondria-mediated ATP production and decelerated telomere shortening, suggesting that nicotinamide could reverse hypermetabolism. Additionally, nicotinamide reduced cellular senescence, corroborating its anti-aging effects. Still, studies testing the effects of boosting NAD+ on cells exposed to glucocorticoid activators are needed.