Key Points:
- Time-restricting feeding (TRF) to 6 hours a day — a type of intermittent fasting — prevents memory loss in Alzheimer’s disease (AD) model mice.
- TRF reduces the number and size of AD plaques, a target for recently approved AD pharmaceuticals.
- Blood glucose levels are reduced and ketone bodies are increased by TRF, suggesting improvements in brain metabolism.
A new study helps to confirm that intermittent fasting isn’t just a fad diet. Researchers from the University of California (UC) San Diego report in Cell Metabolism that TRF prevents memory loss and reduces amyloid plaques in AD mice. Whittaker and colleagues also show that TRF elevates blood ketones — the brain’s fat-derived alternate fuel source — and lowers blood glucose levels. Furthermore, the authors attribute the benefits of TRF to the resynchronization of circadian rhythms.
Time-Restricted Feeding Reverses Alzheimer’s Pathology
To study AD, Whittaker and colleagues experimented with a new AD mouse model called the AAP-KI mouse. APP-KI mice were genetically engineered to have AD-related mutations “knocked-in” (KI) to their DNA within the amyloid precursor protein (APP) gene. APP-KI mice recapitulate AD progression, albeit much faster.
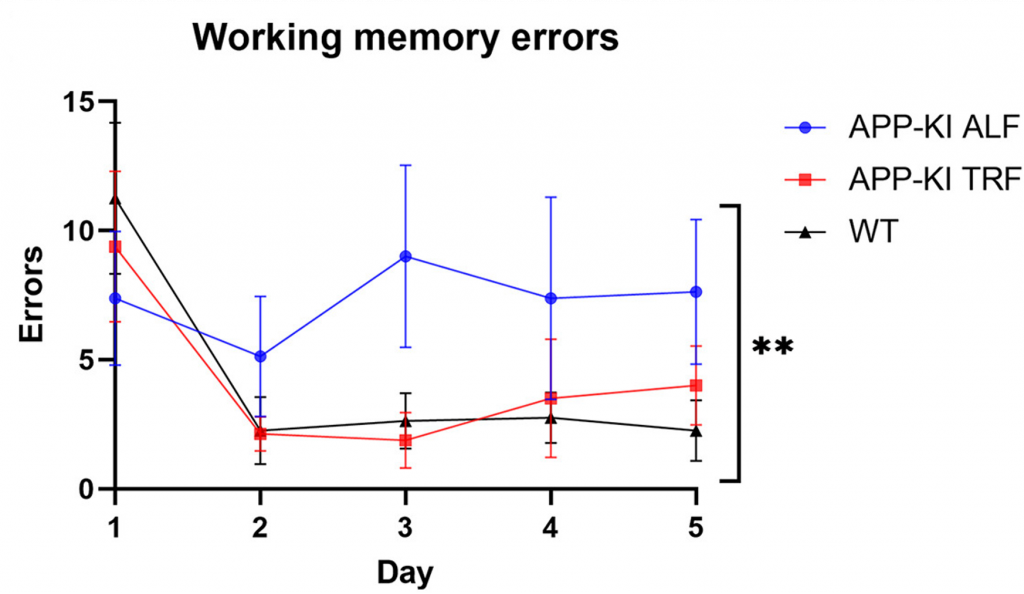
The researchers placed APP-KI mice on a 3-month TRF regimen followed by a memory test involving the 8-Arm Radial Maze. For each test, a mouse was placed at the hub of 8 entryways to corridor-like arms with food at the end, which was eaten when found. However, a memory error was made each time the mouse entered an empty arm, which represents the mouse forgetting they had already eaten the food within that entryway.
After repeating the maze test each day for five days, it was found that APP-KI mice fed a normal diet made more errors than normal mice, suggesting memory loss. However, APP-KI mice on a TRF regimen made just about as many errors as normal mice. These findings suggest that TRF prevents the memory loss exhibited by APP-KI mice.
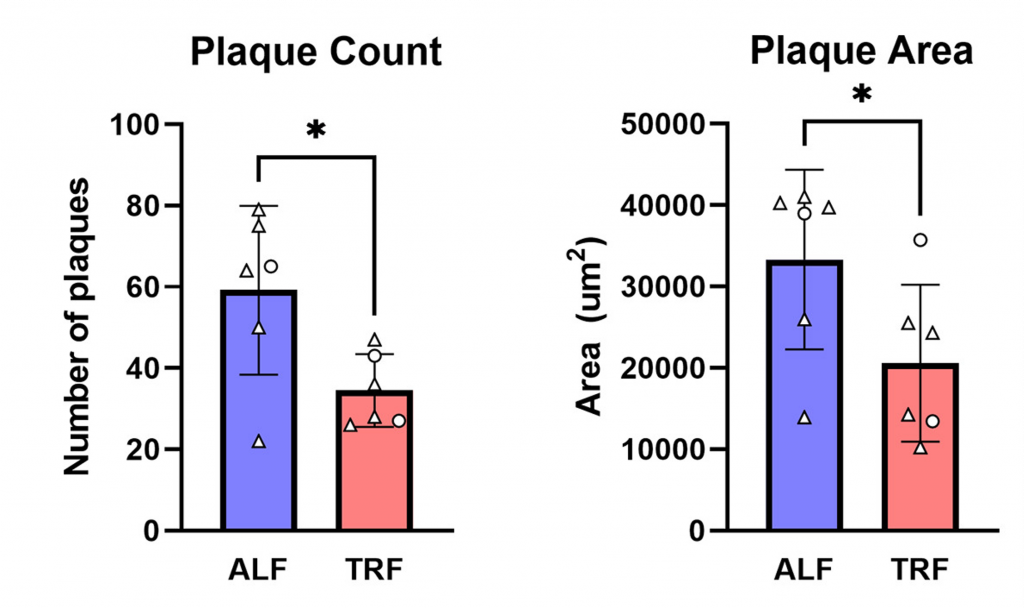
The only two pharmaceutical drugs approved by the Food and Drug Administration (FDA) for the treatment of AD progression — aducanumab and lecanemab — are antibodies developed by Biogen that target amyloid plaques. Amyloid plaques are protein aggregates that build up in the brains of most AD patients and mouse models of AD.
Whittaker and colleagues confirmed the presence of amyloid plaques in the brains of APP-KI mice. Furthermore, they showed that APP-KI mice on a TRF diet had nearly half the plaques as APP-KI mice on a normal diet. Moreover, the plaques were nearly half the size. These findings reveal that TRF may have similar effects to expensive drugs.
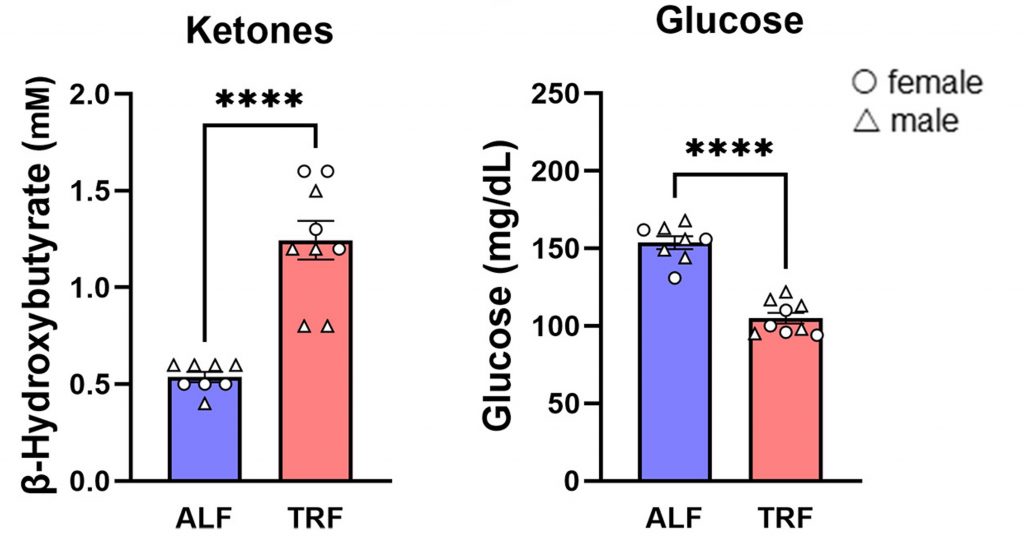
Since TRF and longer durations of fasting are known to improve overall metabolism, including brain cell metabolism, Whitaker and colleagues measured blood glucose and ketone levels. They found that compared to APP-KI mice fed a normal diet, APP-KI mice on a TRF intermittent fasting diet had higher blood ketones and lower blood glucose levels.
These findings suggest that TRF improves brain metabolism, which could contribute to its anti-AD effects. A ketogenic diet (high fat and low carbohydrate), which also elevates ketones, has also been shown to restore memory in AD mice, in addition to countering neurodegeneration. Furthermore, a fasting-mimicking diet improves the memory of AD mice. In both these studies, amyloid was reduced by each corresponding dietary intervention.
The Role of Circadian Rhythms
More than 80% of AD patients experience disturbed sleep and altered circadian rhythms — changes in gene activation that fluctuate in 24-hour cycles and coordinate our physiology and behavior.
“For many years, we assumed that the circadian disruptions seen in people with Alzheimer’s are a result of neurodegeneration, but we’re now learning it may be the other way around — circadian disruption may be one of the main drivers of Alzheimer’s pathology,” said Principal Investigator Dr. Paula Desplats. “This makes circadian disruptions a promising target for new Alzheimer’s treatments, and our findings provide the proof-of-concept for an easy and accessible way to correct these disruptions.”
In addition to the findings presented above, Whittaker and colleagues showed that TRF improved the sleeping patterns and circadian rhythms of AD mice, suggesting that resynchronizing circadian rhythms with TRF contributes to preventing memory loss and reducing amyloid plaques.
“Circadian disruptions in Alzheimer’s are the leading cause of nursing home placement,” said Desplats. “Anything we can do to help patients restore their circadian rhythm will make a huge difference in how we manage Alzheimer’s in the clinic and how caregivers help patients manage the disease at home.”
Importantly, TRF is free of cost and can be implemented at any time.
“Time-restricted feeding is a strategy that people can easily and immediately integrate into their lives,” said Desplats. “If we can reproduce our results in humans, this approach could be a simple way to dramatically improve the lives of people living with Alzheimer’s and those who care for them.”