Key Points:
- Programmed biological tasks to prevent cancer during development (before reproductive age) could promote aging.
- Slowing aging with interventions like rapamycin or dietary restriction essentially slow our growth and development program.
- Cellular reprogramming — a promising anti-aging strategy of the future — could reverse aging by rewinding the developmental software program.
Despite its inevitable reality, at the biological level, aging remains poorly understood. Many theories suppose that aging is caused by the accumulation of damage over time. That is, damage to the “hardware,” including DNA, cells, and organs. However, Dr. João Pedro de Magalhães from the University of Birmingham in the U.K. argues in Genome Biology that aging is not driven by random hardware damage. He proposes that aging is part of a developmental software program optimized for reproduction that becomes detrimental later in life.
Aging Results from Our Biological Software Running Too Long
“Clearly, there is a software program, encoded by the DNA, that is far more advanced, with much greater algorithmic complexity, than any computer program.” – Dr. de Magalhães
How does a single cell (fertilized egg) develop into an organism composed of trillions of cells? While poorly understood, the cell follows instructions written in DNA, telling the cell to execute specific tasks — much like a software program. These biological tasks, in turn, progress the development of a human being. Namely, a human being capable of surviving long enough to reproduce.
The purported flaw in this program becomes apparent when the human being lives beyond reproductive age. Dr. de Magalhães says that biological tasks meant for growth and reproduction are not conducive to healthy aging, as they cause degeneration and dysfunction. He points out that reproductive age is highly correlated with maximum lifespan across species, suggesting that the program isn’t supposed to run too long after we reproduce.

Cancer-Prevention Tasks Could Promote Aging
While it’s possible that running the developmental software program for too long could lead to many age-related diseases, cancer is the major exception. Cancer, caused by DNA damage that tells cells to grow uncontrollably, is a hardware problem. However, the software program includes tasks to prevent the spread of cancerous cells.
Dr. de Magalhães speculates that one cancer-prevention task — one that stops stem cells from propagating — may explain age-related stem cell decline, which underlies diseases like sarcopenia (age-related muscle decline). Stem cells help regenerate and repair damaged tissues, but can potentially be cancerous. Therefore, programmed tasks designed to prevent cancerous stem cells could lead to a loss of much-needed regenerative capacity later in life.
Is Growth the Trade-off for Longevity?
Over 2000 genes have been reported to modulate longevity in model organisms, and manipulating just one of these genes can change the pace of aging. de Magalhães says this supports the idea that aging is a coordinated process. He says,
“There are over 1000 drugs or compounds reported to extend lifespan in model organisms.”
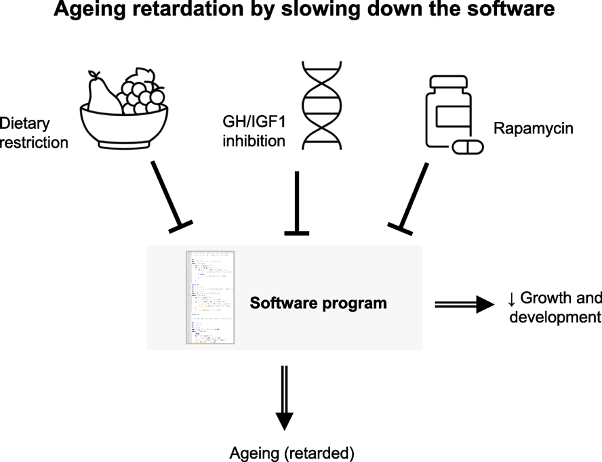
Each of these drugs targets common cellular processes associated with longevity-associated genes, some of which have emerged as modulators of aging. For example, inhibiting the hormone/insulin-like growth factor 1 (GH/IGF1) pathway leads to slower aging. The drug rapamycin also slows the pace of aging. While not a drug, dietary restriction — consuming fewer calories — slows aging as well. What all three of these have in common is that they retard growth and development. Thus, it seems that longevity is achieved at the expense of growth.
Cellular Reprogramming Rewinds the Program
The theory proposed by Dr. de Magalhães is an extension of and another way of looking at the antagonistic pleiotropy theory of aging, originally proposed by George Williams in 1957. Pleiotropy is when a single gene has more than one effect. Thus, antagonistic pleiotropy is when one gene has two or more opposing effects. In the case of aging, a single gene could have a beneficial effect on reproductive success while having a detrimental effect on lifespan.
However, Williams published his theory before we knew of epigenetic regulation — processes cells use to modulate which genes are turned on or off without altering what’s written in our DNA. In fact, the developmental software program de Magalhães speaks of essentially describes the changes in epigenetic regulation that occur over a lifetime. Interestingly, this epigenetic regulation can be manipulated with a new technique called cellular reprogramming.
Cellular reprogramming uses a set of genes called Yamanaka factors to revert cells to an earlier stage of development via epigenetic modulation. In animal studies, cellular reprogramming has been shown to increase lifespan and reverse biological age. Cellular reprogramming has also been shown to make human skin cells thirty years younger. Thus, it’s possible that, within the next decade or two, cellular reprogramming may be implemented to address the “software design flaw” and reverse the aging process.