Key Points:
- Synthetic embryos were successfully grown from mouse embryonic stem cells.
- These embryos reached stages of development that feature a functioning heart.
- The synthetically grown mouse embryos show similar physical and genetic features to naturally grown embryos.
What if you could age gracefully by having new organs provided to us from a Petri dish? A new study suggests that we can manage age-related organ degeneration and disease by replacing our old organs with new organs harvested from synthetically grown embryos.
As reported in Cell, an Israeli research group from the Weizmann Institute of Science used mouse stem cells to grow embryos in a dish. These “synthetic” embryos have distinct similarities in physical appearance and genetic makeup to natural embryos. Remarkably, Hanna and colleagues were able to grow the embryos past the early stages of development and generate a beating heart.
“Cells and tissues from such synthetic advanced organized entities could be potentially useful for cell differentiation research and transplantation biotechnology,” the investigators wrote.
Embryos Grown from Embryonic Stem Cells
We are made up of many different cell types, such as brain cells, muscle cells, and skin cells. Embryonic stem cells are capable of becoming any cell type, which is how embryos eventually develop into adults. Hanna and colleagues took groups of embryonic stem cells from mice and experimentally activated specific genes to induce an aspect of embryonic development within each group.
The investigators found that when grown together, these different stem cell groups work in concert to give rise to cell types that ultimately become different parts of an embryo. While not perfect, approximately 25% of the cells grow in the correct spatial orientation. After five days of development, the synthetic embryos are moved to an incubator to optimize growth conditions. In the incubator, the embryos develop early-stage organs, something that has not previously been accomplished outside of a uterus.
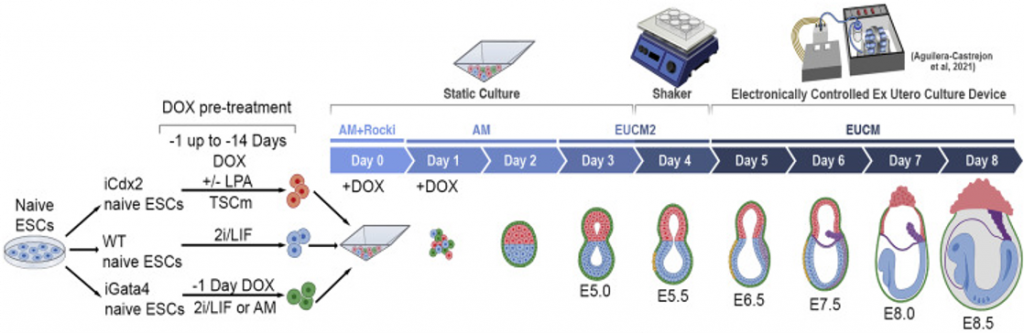
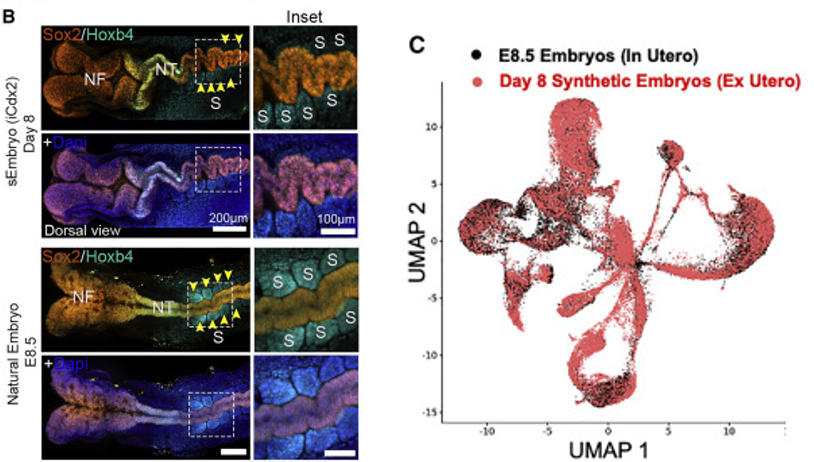
During development in the incubator, the synthetically grown embryos undergo changes in shape (morphologically) similar to natural embryonic development. By day eight, the embryos develop the early stages of a head, the early stages of a brain and spine (neural tube), and a beating heart. Furthermore, genetic analysis reveals that day eight embryos are remarkably similar to natural day 8.5 embryos, despite some minor differences in appearance.
Can we Grow Human Organs in a Dish?
As we age, our organs give out and no longer function properly, leading to disease or death. Organ transplants are an option, but organ transplant lists have long wait times, and there are donor-recipient compatibility limitations. If we could grow organs from human embryonic stem cells – which can be obtained from leftover embryos that are not used (and would likely be discarded) during fertility treatments – organs would be more readily available. With this in mind, organs could be replaced before they fail, preventing disease and extending healthspan – the amount of time that we can live healthy lives.
There are, however, ethical issues pertaining to the use of human embryonic stem cells. This issue may be circumvented by the use of induced pluripotent stem cells. Induced pluripotent stem cells are adult cells like skin or blood cells that can be induced into pluripotency (the ability to differentiate into many cell types), making them like embryonic stem cells. This means that our own skin cells could potentially be used to grow new organs.
Synthetically developing mouse embryos with functioning hearts is a scientific feat. This technology could be used to further our understanding of embryonic development and, as mentioned above, could potentially be used to grow human organs. However, Hanna and colleagues found that not all stem cells developed into later-stage embryos. This indicates that more research into efficacy and optimization may be needed. In any case, this is definitely a step in the right direction toward synthetic organs becoming a reality.