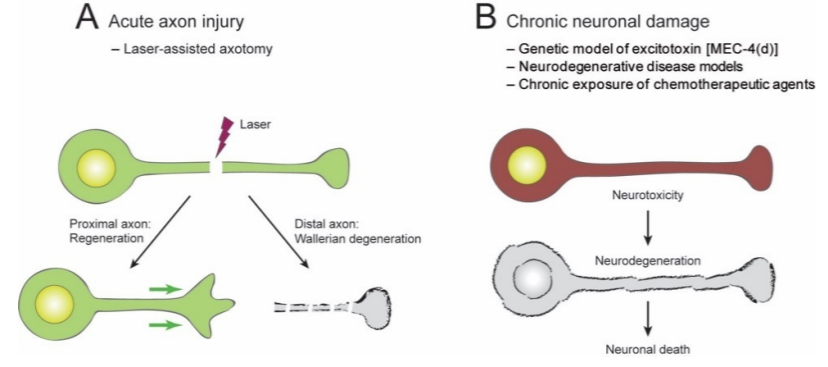
Traumatic brain injuries and disorders of the brain, such as Alzheimer’s disease and Parkinson’s disease, induce neuronal damage. Neuronal damage induced by injuries and diseases can become present in the short-term right after the occurrence, or it can develop chronically over time. Researchers investigate brain injuries and diseases utilizing different animal models with the ambition to develop effective therapeutics for both short-term and long-term neuronal damage.
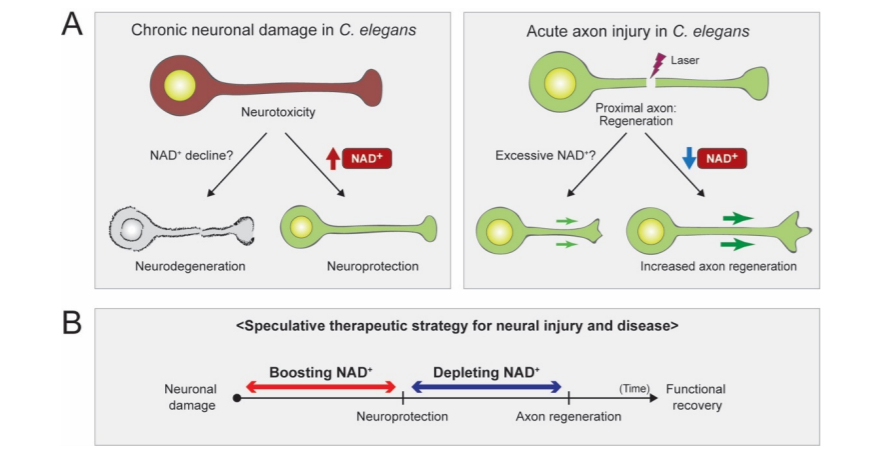
In a review article published in Biomolecules, Kim and colleagues from Hallym University in Korea presented their findings on the involvement of the essential coenzyme nicotinamide adenine dinucleotide (NAD+) and its precursors in the impairment of neurons in roundworms (C. elegans). Investigators postulated that elevated levels of NAD+ can prevent neuronal damage, while diminished levels of NAD+ can induce the generation of axons, neuronal regions that pass on cellular signals. In addition, they evaluated whether controlling NAD+ at precise time intervals in various tissues can halt neurodegenerative conditions and neuronal damage caused by injuries and neural conditions.
NAD+ facilitates many cell processes, ranging from generating energy for the body through metabolic pathways to DNA repair, cell signaling, and gene expression. Evidence suggests that compounds that synthesize or utilize NAD+ provide many critical functions that promote the protection of neurons and regeneration of axons. Scientists have employed roundworms to explore the impact of controlling NAD+ in neurons because humans and roundworms exhibit the same chemical components required for synthesizing and modulating NAD+.
The review article presented some current findings from NAD+ research that utilized roundworms. Furthermore, they showed that experiments investigating short-term axon injury suggested that elevated levels of NAD+ fail to mitigate axon degeneration. Scientists have utilized roundworms with genetic alterations that boost the levels of NAD+ in neurons, which failed to demonstrate protection against axon degeneration from axonal injury caused by a microscopic laser.
The review article proceeded with discussing their investigations into long-term neuronal damage, and their findings indicated that increased levels of NAD+ contribute to the protection of neurons, thus guarding the cell body. When scientists elevated NAD+ concentration by controlling the levels of a crucial compound in NAD+ biosynthesis, NMNAT/NMAT-2, they discovered that increased levels of NAD+ promoted neuroprotective properties in roundworms with damaged neurons.
The data presented in the study suggests that enzymes called PARPs, which rely on NAD+ to operate, are involved in a biological mechanism that inhibits the regeneration of axons when the levels of NAD+ are elevated. Interestingly, investigators noticed that mutant worms displayed improved aptitude in regenerating axons upon inhibition of PARP. Furthermore, the investigators hypothesized that diminished levels of NAD+ with alterations in compounds that synthesize NAD+ did not stimulate the inhibitory effect of PARP enzymes on the regeneration of axons. Thus, their theory suggests that decreased levels of NAD+ would induce axon regeneration.
“As we come to understand NAD+ biology in greater detail, we may need to regulate the NAD+ levels in proper time frames and in specific tissues,” stated Kim and colleagues in their review. After neuronal damage occurs, it may be possible to mitigate the effects of damaged neurons with high doses of NAD+, and reducing NAD+ later may provide support in the regeneration of axons. In addition, a thorough understanding of NAD+ biology could aid the generation of effective therapeutics that target conditions induced by damaged neurons.