Key Points:
- Elamipretide restores cardiac and skeletal muscle performance in aged mice by improving mitochondria and reducing inflammation.
- Elamipretide improved physical function in aged mice without lowering biological age, which was estimated using molecular markers in DNA and gene activity.
An estimated 6.7 million adults in the United States are currently living with heart failure, a condition that becomes increasingly common with age. Projections suggest that this number will rise steadily over the coming decades, reaching roughly 8.7 million by 2030 and over 11 million by 2050. At the same time, skeletal muscle mass declines by up to 30% between early adulthood and old age, contributing to frailty, reduced mobility, and a loss of independence. These declines in heart and muscle function are often driven by the same underlying problem—mitochondrial dysfunction, which impairs energy production and increases cellular stress.
One compound known to target this problem is elamipretide. In a new study published in Aging Cell, researchers explored whether this mitochondria-targeted molecule could restore lost performance in aging muscle and heart tissue. Remarkably, they found that elamipretide significantly improved both cardiac and skeletal muscle function in old mice. However, these gains occurred without reversing the molecular signatures of aging typically measured by epigenetic and transcriptomic clocks, which estimate biological age based on changes in DNA and gene activity that tend to accumulate over time. Taken together, the findings suggest that enhancing mitochondrial health may improve physical resilience in older organisms, even if the biological age of their tissues remains unchanged.
Elamipretide Improves Muscle and Heart Function
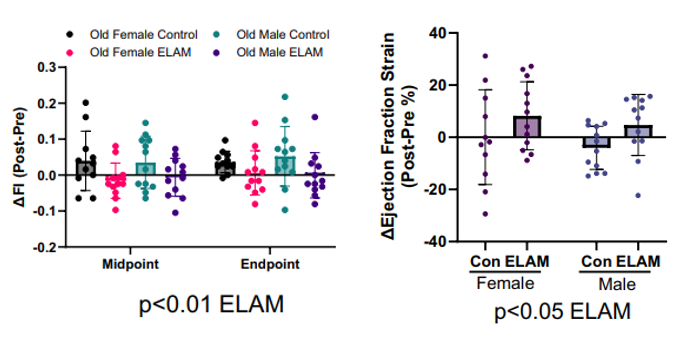
To investigate whether elamipretide could counteract physical decline in aging, researchers at the Salk Institute treated 24-month-old male and female C57BL/6J mice. This age corresponds roughly to 69-year-old humans. The mice were treated for eight weeks using small implanted devices that steadily released the drug into their bodies. A separate group of 5-month-old mice served as young controls to help benchmark performance and molecular aging.
Throughout the study, the researchers assessed frailty scores, cardiac performance, and muscle strength and endurance. As expected, aged mice exhibited higher frailty indices, weakened heart function, and reduced physical performance compared to their younger counterparts. However, elamipretide treatment significantly improved several of these parameters. In the heart, treated mice showed enhanced systolic function, referring to the heart’s ability to contract and pump blood effectively with each beat. Diastolic function, which governs how well the heart relaxes and fills with blood, remained unchanged, as did measures of cardiac hypertrophy (growth).
In skeletal muscle, elamipretide helped aging mice generate force more efficiently and resist fatigue during repeated contractions. The most pronounced improvements occurred in older female mice, who started with more severe baseline deficits. Following treatment, these mice recovered strength faster and maintained it for longer periods, suggesting that elamipretide may restore not just muscle power but also endurance in aging muscle tissue.
Biological Age Stays the Same, but Key Longevity Pathways Shift
To assess whether elamipretide had any impact on the biological age of tissues, researchers examined changes in DNA methylation and gene activity in the heart and skeletal muscle. These are common molecular markers used in aging research. The analysis relied on molecular aging clocks, which are tools that estimate how old a tissue appears biologically by measuring patterns in DNA and gene activity that tend to shift with age.
While these clocks clearly distinguished aged tissue from young, elamipretide treatment did not meaningfully alter the predicted biological age of heart or skeletal muscle in older mice. A small reduction was observed in transcriptomic age in young male hearts, but this effect did not extend to the older animals, which were the primary focus of the study.
However, when researchers looked beyond age prediction to examine broader shifts in gene activity, they observed more encouraging results. Elamipretide increased the activity of pathways tied to mitochondrial energy production, fatty acid metabolism, and oxidative phosphorylation. These same pathways are typically suppressed with age. At the same time, the drug downregulated inflammatory and immune-related processes such as TNF-alpha and interferon signaling, which are often elevated in aging tissues.
These effects were particularly pronounced in the hearts of older mice and were consistent with gene expression signatures associated with extended lifespan across mammalian species. So, while the drug did not roll back the molecular clock, it did appear to recalibrate the transcriptome in ways that support healthier aging at the cellular level.
A Different Path to Rejuvenation?
As interest in longevity science grows, many interventions are judged by their ability to reverse molecular hallmarks of aging. However, this study suggests that physical function may offer a more practical benchmark. Elamipretide improved endurance, strength, and cardiac performance in aged mice without altering their biological age. At the same time, it reactivated pathways tied to mitochondrial efficiency and suppressed inflammatory processes that typically worsen with age.
These effects point to a different model of aging intervention—one focused on preserving the body’s performance and resilience rather than resetting its molecular clock. In this light, aging may be less about turning back time and more about reinforcing the systems that keep us upright, active, and independent as the years go on.