Key Points:
- Analysis of obstructed kidneys from pediatric patients uncovered aberrant NAD+ metabolism.
- CD38, an NAD+-consuming enzyme, was strongly induced in pediatric patients and animals with obstructive nephropathy.
- NAD+ supplementation or CD38 deletion or inhibition mitigated obstruction-induced kidney damage in animals.
Researchers from Beijing demonstrated that a leading cause of kidney injury in pediatric patients is tied to the dysregulation of NAD+. In pediatric patients with obstructive nephropathy, the enzyme CD38, which drains NAD+ supplies, was highly upregulated, and when the researchers blocked CD38 or supplement NAD+ in mice with obstructed kidneys, they observed significantly reduced kidney damage. Published in Molecular & Cellular Proteomics, this article suggests that NAD+ boosting might represent an optional therapy for obstructive nephropathy.
Nailing Down NAD+ and Nephropathy
Obstructive nephropathy is a leading cause of kidney injury in infants and children and can lead to serious long-term kidney disorders, such as chronic kidney disease and renal fibrosis. But effective therapies are still lacking; therefore, it is critical to expand our understanding of obstructive nephropathy to facilitate the development of new treatments.
The kidney (and the liver) appear to act as a hub for whole-body NAD+ homeostasis. Research shows that all enzymes involved in NAD+ biosynthesis, with only a few exceptions, are highly expressed in these two organs.
A rapid decrease in NAD+ levels is observed in acute kidney injury, which is partially driven by impaired NAD+ biosynthesis. Animal kidney injury models have shown that restoring NAD+ levels by giving the body NAD+ precursors like nicotinamide mononucleotide (NMN) or nicotinamide (NAM) protects against kidney damage. Several animal studies and clinical observations also show that there is a link between the way NAD+ is used and the development of chronic kidney diseases. But there aren’t many details about NAD+ metabolism, its roles, and how it works in chronic kidney diseases like obstruction-induced renal fibrosis.
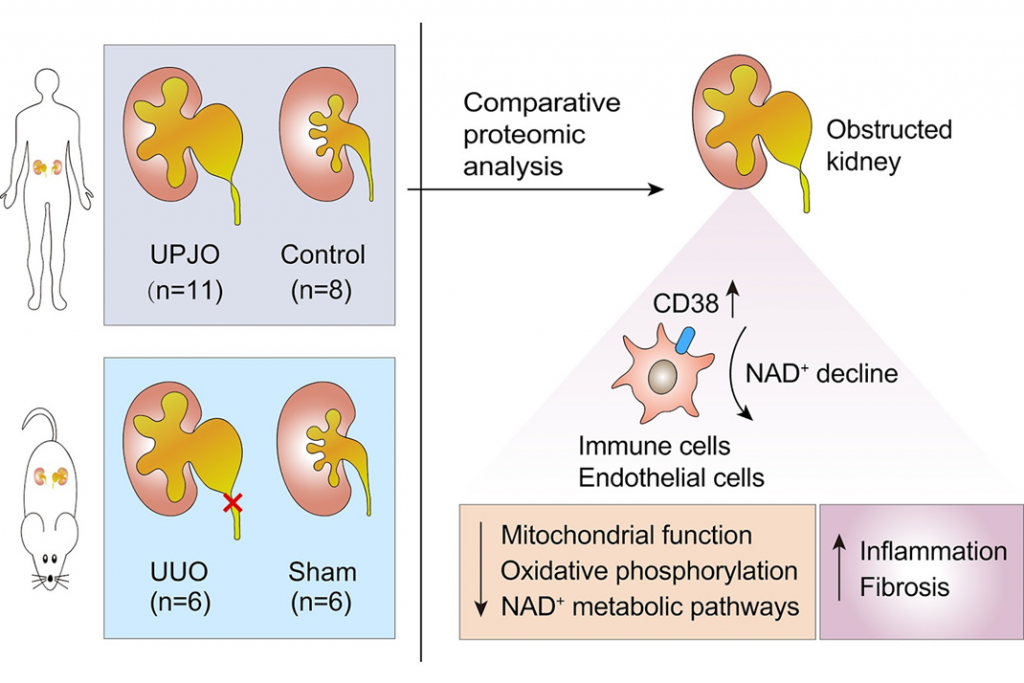
In the current study, a team of researchers from several institutions in Beijing—including the University of Chinese Academy of Sciences and various Medical Centers of Chinese PLA General Hospital—examined the protein profile of human-obstructed kidneys. Importantly, the protein profiling, which is called proteomics, found a problem with the way NAD+ is used in clogged kidneys that had not been looked into before.
NAD+ Boosting Reduced Kidney Injury
Moreover, the major NAD+-consuming enzyme CD38 was strongly induced in human and experimental obstructive nephropathy, which led to declining renal NAD+ levels. Numerous cell types express CD38, and inflammatory conditions can induce its expression, particularly in hematopoietic cells. This study found that CD38 was partially expressed in immune cells in animals with kidney injury. Half of these cells were white blood cells called macrophages that emerged during the fibrotic process. This isn’t the first study to report that CD38 on macrophages is capable of reducing tissue levels of NAD+ during aging and the role of CD38 in modulating inflammation by regulating key immune cell processes.
What’s unique to this study is the role of CD38 on NAD+ levels during kidney injury. In a model of renal fibrosis, taking out or blocking CD38 and giving the animals more NAD+ also helped. This helped in part by reducing inflammation in the kidneys. For the first time, this study gives solid molecular evidence that comes directly from children with obstructive nephropathy.
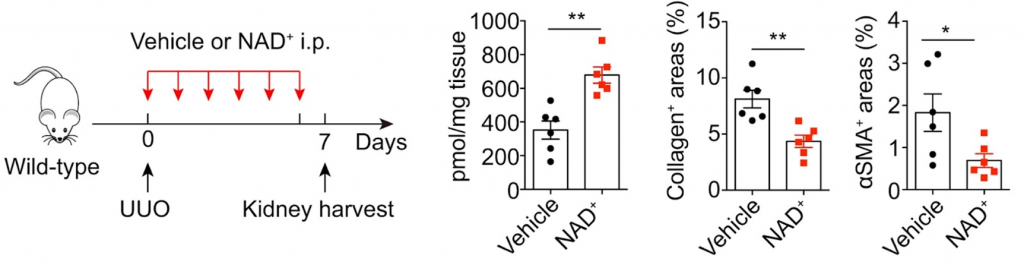
This article suggests that NAD+ boosting might represent an optional therapy for obstructive nephropathy. Supplementing with NAD+ has been shown to help in a number of models of kidney disease and injury. In animal models of acute kidney injury, increasing NAD+ levels with NMN or NAM stops kidney damage, and treating kidney cells with NMN lowers the expression of fibrotic factors. NA and NAM have been shown to alleviate several complications of chronic kidney disease. Boosters of NAD+ are being tested in humans with kidney diseases in a number of clinical trials that have had mixed results so far.