Multiple sclerosis is a seriously disabling disease involving our immune system’s attack on healthy cells. This self-inflicted response progressively causes nervous system inflammation and the stripping of protective fats coating nerve projections (demyelination). Figuring out how to suppress this nervous system inflammation and keep nerves healthy would be an attractive therapeutic strategy.
Wang and colleagues published a study in International Immunopharmacology where they treated mice with nicotinamide adenine dinucleotide (NAD+) that improved nervous system function in multiple sclerosis. NAD+ substantially reduced brain damage and delayed neurological symptom onset in in mice modeling multiple sclerosis. This treatment also helped to alleviate demyelination and reduce inflammatory factor levels in the spinal cord. Findings from the study highlight NAD+ as a possible therapeutic strategy for treating multiple sclerosis.
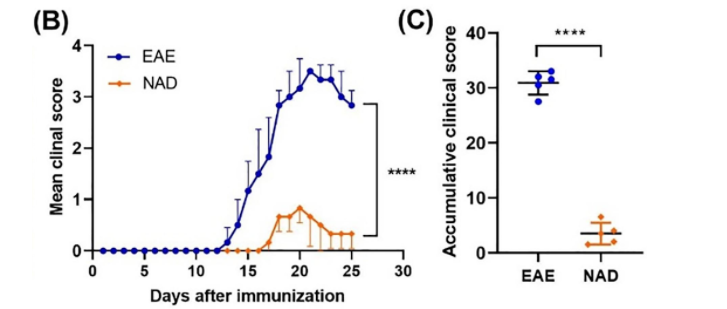
NAD+ Treatment Improved Neurological Function in Mice with EAE
In their study, Wang and colleagues found that NAD+ significantly delayed neurological symptom onset in mice with autoimmune encephalomyelitis (EAD), a multiple sclerosis model. EAE mice injected with NAD+ (250 mg/kg) showed substantially lower scores on tests evaluating the clinical severity of neurological function. The NAD+-treated EAE mice had healthier white matter, where nerves wrapped in fat tissue travel to the nervous system.
“We demonstrated that NAD+ can significantly reduce the functional severity and white matter damage caused by experimental autoimmune encephalomyelitis in mice,” said the researchers in the article.
NAD+ Treatment Alleviated Demyelination and Spinal Cord Inflammatory Factor Levels
Wang and colleagues saw that the activation of inflammation-associated nervous system cells called microglia was inhibited in NAD+-treated EAE mice. These mice showed reduced nervous system inflammation and demyelination. Along these lines, the EAE mice spinal cords had infiltration with inflammatory cells along with massive injuries and demyelination. However, in NAD+-treated EAE mice, the levels of infiltrating inflammatory cells were significantly reduced with fewer signs of demyelination and damage to neuron connections.
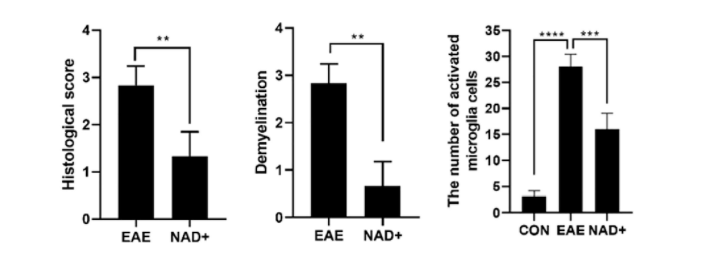
The research team thinks that NAD+ treatment alleviated EAE’s detrimental effects in part by downregulating the presence of inflammation-associated molecules called cytokines. For example, they saw that NAD+ substantially reduced levels of the inflammation markers NLRP3 and IL-17 in the EAE mouse spinal cord. This could lead to the prevention of large scale immune cell infiltration to the nervous system’s white matter, which may limit the severity of the disease.
Inhibition of Autophagy Aggravated Clinical Symptoms and Worsened Inflammation and Demyelination
In addition to reducing NLRP3 levels and injury from demyelination, NAD+ promoted increased levels of proteins involved in autophagy—the process cells use to remove unnecessary or dysfunctional components for degradation and recycling.
“We show that induction of autophagy is essential for these NAD+-induced benefits, at least in part by suppressing the inflammasome and downstream pro-inflammatory cytokine signaling.”
This is supported by other studies showing that autophagy blocks inflammation to prevent the harmful amplification of inflammatory molecules that promote demyelination.
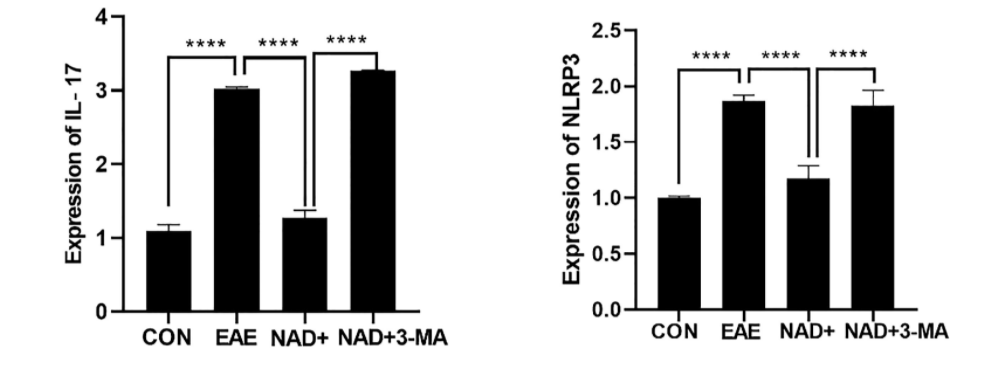
Blockade of Autophagy Attenuated the Protective Effects of NAD+ in EAE Mice
Dysregulated autophagy can prolong inflammatory responses, possibly leading to autoimmune or inflammatory diseases. Along these lines, when Wang and colleagues blocked autophagy with the chemical 3-MA, they saw aggravated clinical symptoms, worse inflammation and demyelination, and attenuated protective effects of NAD+ in EAE mice. Inflammatory cell infiltration was significantly enhanced in mice treated with the autophagy-blocking 3-MA and NAD+ compared to those treated only with NAD+. Moreover, the reduced levels of inflammation markers NLRP3 and IL-17 in the spinal cords of NAD+ treated EAE mice were significantly reversed by 3-MA.
“Our results indicate that NAD+ suppresses the NLRP3 inflammasome at least in part through the activation of autophagy to relieve the symptoms of experimental autoimmune encephalomyelitis,” said the researchers in the article.
Future Directions of NAD+ Treatment in Multiple Sclerosis
These findings suggest that autophagy regulation by NAD+ treatment may be an effective therapeutic strategy for multiple sclerosis. More research is needed to unravel how autophagy suppressed neuroinflammation affects multiple sclerosis. Importantly, clinical trials testing the effectiveness of NAD+ treatment in multiple sclerosis patients are necessary to see if this therapeutic strategy can help humans.