Key Points:
- Microscopic sacs secreted by stem cells called exosomes reverse cellular senescence, which was once thought unfeasible.
- The exosomes prolonged the lifespan of mice while improving their physical performance and cognition.
- The exosomes carry miR-302b particles, which drive their age-delaying effects.
Mayo Clinic’s conceptual framework for treating the most deadly age-related diseases, including cancer and cardiovascular disease, involves targeting a single shared biological factor — senescent cells. Until now, the most well-established way of reducing senescent cells has been senolytics — compounds that selectively eliminate senescent cells.
However, challenging the dogma that senescent cells are irreversible, Bi and colleagues have discovered how to reverse the senescence state with microRNA (miR)-302B. Moreover, miR-302B, which shuts off specific genes, prolongs the lifespan of mice while improving their cognition and physical performance. These findings have far-reaching implications for human therapies that prevent or treat age-related diseases.
Regenerating the Body One Cell at a Time
Aging is characterized by the gradual degeneration of our body, which is offset by our capacity to regenerate. Both occur at the cellular level, whereby losing cells degenerate our organs, and gaining cells regenerate them. Organs like the liver, pancreas, and lungs can regenerate through proliferation — the increase in cell numbers resulting from cell division, whereas organs like the skin and intestine rely more on stem cells.
Stem Cells
Stem cells are unspecialized cells that can differentiate (transform) into specialized cells, such as skin cells or intestinal cells. When stem cells divide, one copy can remain a stem cell while the other can differentiate into a specialized cell. While organs like the skin and intestine regenerate continually with the help of stem cells, we have only recently discovered that organs like the brain, and potentially even the heart have the capacity to regenerate via stem cells.
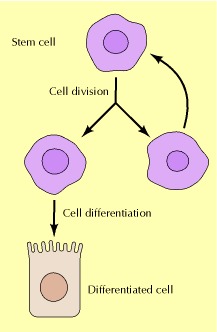
While our cells can die for many reasons, cell proliferation and stem cells are the primary means for generating new ones. However, our stem cells lose their regenerative capacity with age and cellular proliferation generates senescent cells.
Senescent Cells
Cell senescence has traditionally been described as a state of irreversible cell proliferation arrest but this is a poor description originating from research done in the early 1960s. Many of our cells, such as neurons and heart cells, do not proliferate, meaning they are already in a state of cell proliferation arrest. While our neurons and heart cells can potentially become senescent cells, they are normally not senescent. Moreover, as Bi and colleagues have found, the senescent state is reversible. Thus, the traditional description of senescent cells is incomprehensive and outdated.
Cell senescence can better be described as a dysfunctional state that promotes organ degeneration. When our cells encounter a stressful stimulus, such as DNA damage, they enter a senescent state. Senescent cells secrete molecules known as SASP (senescence-associated secretory phenotype) factors, including pro-inflammatory molecules and tissue-degrading molecules. With age, senescent cells accumulate throughout the body and contribute to age-related conditions, including neurodegeneration, cardiovascular disease, lung disease, diabetes, and liver disease.
So far, one of the best ways to remove senescent cells is with senolytics. However, each cell lost contributes to degenerative aging. Therefore, by killing senescent cells, senolytics may only halt the progression of aging. If senescent cells can be turned back into normal cells (if cellular senescence was reversible), then no cells would be lost and the progression of degenerative aging could be reversed.
Exosomes Reverse Cellular Senescence
Stem cell-derived exosomes — membrane-bound vesicles secreted by cells — appear to have similar effects to stem cells. Exosomes are microscopic sacs that carry various molecules such as proteins and microRNAs — short single strands of RNA that turn off genes. By traveling through the bloodstream, exosomes can deliver their cargo to any cell in the body. Mounting evidence has demonstrated that stem cell-secreted exosomes provide similar clinical benefits to stem cell therapy. With this in mind, Bi and colleagues chose to explore the regenerative potential of exosomes by testing how they influence senescent cells.
To determine if the senescent state is reversible, Bi and colleagues employed human skin cells, which proliferate throughout a lifetime. It was in skin cells that senescence was first discovered, whereby it was shown that skin cells can only replicate a limited number of times before becoming senescent cells, a phenomenon now known as replicative senescence.
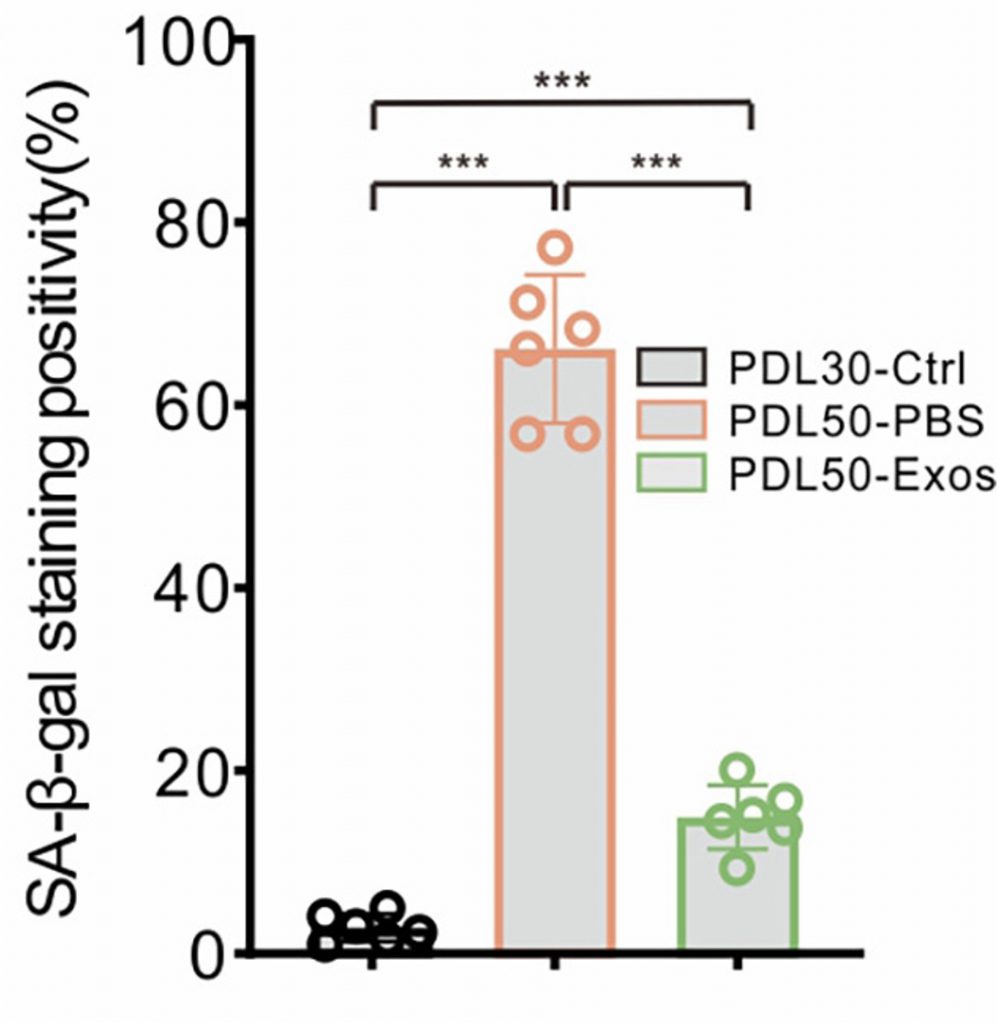
Hence, to trigger senescence, the researchers replicated the skin cells 50 times. They then exposed the senescent skin cells to exosomes derived from embryonic stem cells. As a result, they found that exosome treatment significantly reduced the number of senescent cells. Moreover, the exosome treatment led to a reduction in senescence- and SASP-related genes. Additionally, exosome treatment significantly increased cell proliferation, demonstrating that the exosomes reversed cell proliferation arrest.
Exosomes Delay Aging by Delivering miR-302b
Upon finding that stem cell-derived exosomes reverse cellular senescence, Bi and colleagues injected these exosomes into mice aged 20 months, approximately equivalent to 60 human years. Remarkably, this prolonged the lifespan of the mice and ameliorated aspects of aging such as gray hair and wrinkles. The exosome-injected mice also showed improvements in balance, grip strength, and cognitive ability.
Importantly, the proportion of senescent cells was reduced in the kidney, liver, lung, spleen, and skin of the exosome-treated mice. This was accompanied by a reduction in pro-inflammatory molecules, which are often secreted by senescent cells as part of the SASP. Moreover, proliferation was restored in the liver and skin, suggesting the restoration of youthful organ regeneration.
miR-302b
In seeking which cargo harbored by the exosomes contributes to age deferral, Bi and colleagues came to a microRNA species called miR-302b. In general, microRNAs bind to the mRNA transcripts of genes, initiating the degradation of those transcripts, and blocking their translation into proteins. As gene regulators, microRNAs play a key role in development, physiology, and disease. Indeed, Victor Ambros and Gary Ruvkun won the 2024 Nobel Prize in Physiology or Medicine for discovering microRNA.
Bi and colleagues found that miR-302b blocks genes involved in cell division and DNA damage repair. Specifically, miR-302b was found to block two genes that initiate cell proliferation arrest. They then repeated many of the same experiments conducted with the stem cell-derived exosomes but this time with miR-302b. Interestingly, to do so, they filled human kidney stem cells with excess levels of synthetic miR-302b, calling them Exos-302B.
This time, 25-month-old (roughly 70 human years) mice were injected with Exos-302B. This led to restored hair growth, which had fallen out from natural aging. Similar to the embryonic stem cell-derived exosome treatment, Exos-302B treatment improved balance, grip strength, and cognition while reducing inflammation and the proportion of senescent cells in various organs. Moreover, the cell proliferation arrest genes were repressed in the liver, skin, and brain, suggesting proliferation restoral.
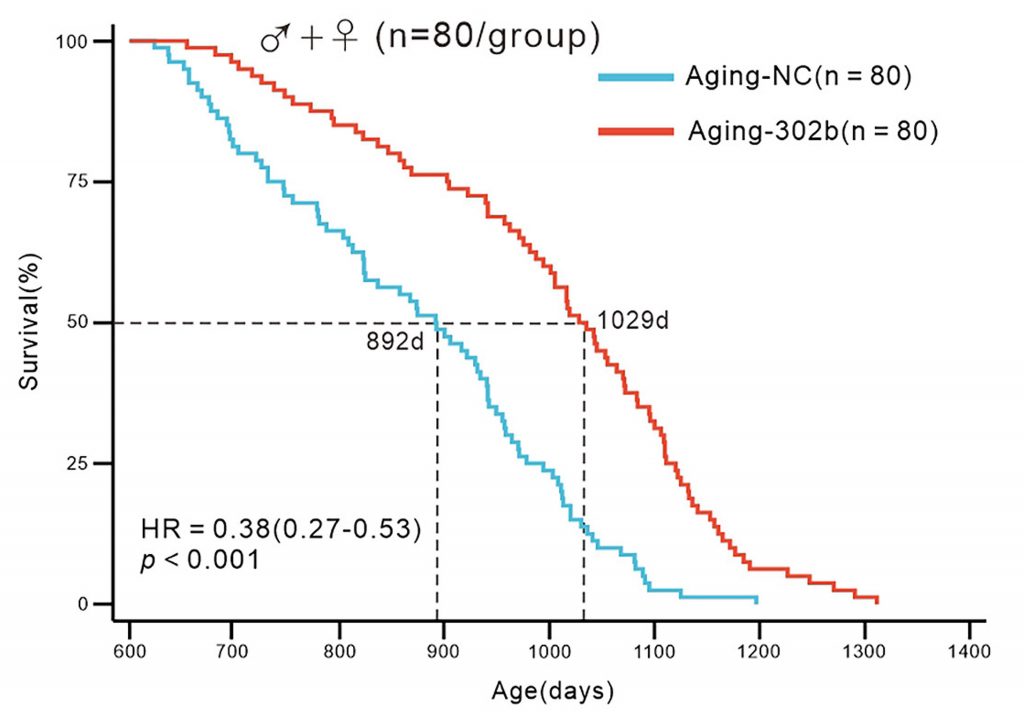
In the end, the median lifespan of the Exos-302b-treated mice was extended by about 15.4%. Furthermore, the risk of death was reduced by 38%. When separating the data by sex, the lifespan of male mice was increased by 22.1% and the lifespan of female mice was increased by 14.9%. Additionally, the Exos-302b-treated mice showed no differences in tumor or disease burden, suggesting the therapy is safe and does not trigger tumor growth.
“Collectively, long-term administration of miR-302b extended the lifespan of aging mice, ameliorated visible signs of aging, did not increase tumor or disease burden, reversed the decline in cellular proliferative capacity within aging tissues, alleviated aging-related transcriptomic signatures, and thereby effectively decelerated the aging process,” the authors concluded.
Does miR-302b Really Only Target Senescent Cells?
Leading longevity researcher David Sinclair, PhD, posits that aging is rooted in gene expression changes triggered by alterations in the three-dimensional structure of DNA called epigenetics. However, the epigenetic changes that underlie aging can be reversed using a process called reprogramming, whereby cells are reverted to an earlier stage of development. Reprogramming has been shown to prolong the lifespan of mice by 109%.
Furthermore, research has shown that miR-302b is involved in reprogramming, suggesting it may play a role beyond reversing senescence. While Bi and colleagues say that miR-302b’s age-delaying effects are “primarily attributed to the restoration of cell proliferation rather than [reprogramming],” they did not conduct thorough experiments to confirm this. Therefore, it is possible that, in addition to reversing cellular senescence, miR-302b exerts its rejuvenating effects through reprogramming.