Key Points:
- Food supplementation with the NAD+ precursor nicotinamide riboside (NR) protected neurons linked to Parkinson’s in mice, preventing the development of Parkinson’s-like behaviors.
- These results support the clinical research of NAD+ precursors on neurodegeneration by offering a mechanistic understanding of how these molecules may help older adults at risk for Parkinson’s disease.
New research indicates a small step has been made towards finding a cure for conditions like Parkinson’s. According to a study, NAD+ precursors may shield dopaminergic neurons from degeneration, which results in Parkinson’s disease. The development of Parkinson’s disease was stopped when scientists at Zhejiang University School of Medicine in Hangzhou, China, added NR to the diets of mice. In contrast, Parkinson’s-like behaviors in mice were amplified by blocking the production of NAD+. According to this study, which was published in the journal Brain Research Bulletin, NAD+ precursors may be able to prevent dopaminergic neurons from degenerating, which could lead to a treatment strategy for Parkinson’s disease.
Drivers of Parkinson’s brain cell degeneration
The center of your brain contains a complex network of nerve “highways” that are necessary for many vital behaviors, including mood regulation, movement control, and other cognitive processes. One connection point, the substantia nigra, is home to a particular kind of brain cell that generates dopamine, the chemical that gives us “reward” and “pleasure.” People commonly develop Parkinson’s disease (and sometimes drug addiction or other psychiatric disorders) when these brain cells, called dopaminergic neurons, are damaged or start to function abnormally.
The mainstay of modern Parkinson’s treatment is levodopa, a symptom-relieving drug that replaces dopamine lost in the substantia nigra. Still undiscovered, though, is a Parkinson’s disease treatment that can reverse the underlying causes of certain brain cell neurodegeneration in the substantia nigra. Therefore, figuring out what causes dopaminergic neurodegeneration is essential to creating therapies that can halt or slow the progression of Parkinson’s disease.
Numerous factors, such as aging, genetics, toxins, and biological factors, have been found to contribute to abnormal or degenerating dopaminergic neurons. More is known about the mechanisms in dopaminergic neurons that these factors ultimately impact, including mitochondrial dysfunction and oxidative stress, which play a role in the onset and progression of Parkinson’s disease. When dangerous molecules (ROS) build up as a result of an imbalance in their production and elimination, it is known as oxidative stress. This leads to cell damage and the loss of dopamine neurons. Additionally, oxidative stress is made worse by mitochondrial dysfunction, creating a vicious cycle that hastens the death of neurons.
Blocking Parkinson’s in mice with NAD+
Interestingly, numerous studies have demonstrated that NAD+, which has been demonstrated to diminish with age and typically correlates with aging-related diseases like neurodegenerative diseases, is essential for enzymes that can regulate oxidative stress. In a similar vein, research has demonstrated that NAD+ precursor supplements can help shield mitochondria, lower oxidative stress, and avert a number of health problems, especially in brain cells. For instance, nicotinamide mononucleotide (NMN) helps to lessen oxidative brain damage, while NR has been demonstrated in mice to enhance memory and brain function in aging and Alzheimer’s disease.
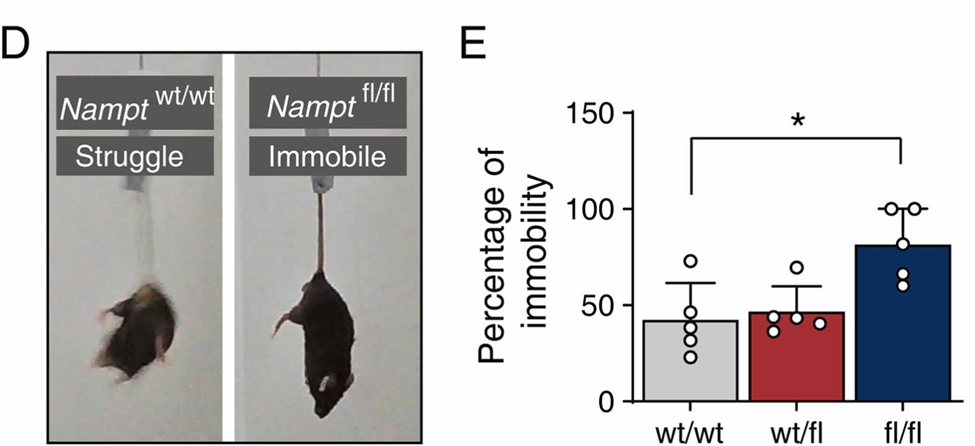
This led scientists in Wei-Ping Zhang’s lab to investigate the relationship between NAD+ production, oxidative stress, and Parkinson’s disease development. They found that dopamine-producing substantia nigra neurons generally have higher levels of NAMPT, an enzyme essential for the synthesis of NAD+, than other dopamine-producing neurons in the same region. When NAMPT was deleted in the substantia nigra, dopamine-producing neurons degenerated, resulting in Parkinson-like symptoms in the mice, including impaired and restricted movement.
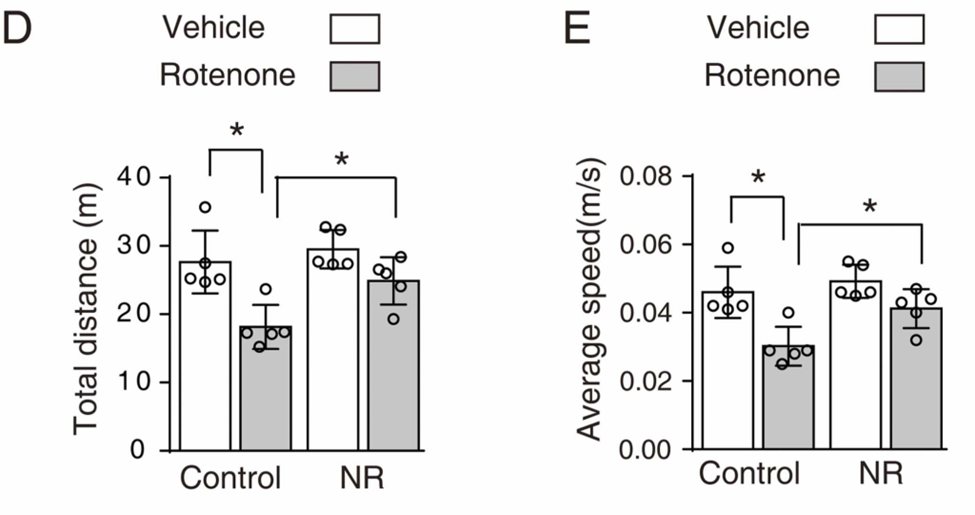
The scientists then looked into whether boosting NAD+ synthesis could reverse the effects of oxidative stress on the substantia nigra’s dopamine-producing neurons. They achieved this by injecting rotenone, an oxidative stressor that has been shown to induce Parkinson’s disease in mice, directly into the substantia nigra for thirty days in a row. By supplementing their diet with 400 mg/kg/day of NR, a NAD+ precursor, half of these mice were shielded from the loss of dopamine-producing neurons, which also correlated with preventing the onset of impaired movement. By reproducing these results in human cells that had previously been used to simulate Parkinson’s disease, the researchers showed that NAD+ precursors enhanced cell survival and decreased oxidative stress.
Testing NAD+ precursors in Parkinson’s Patients
Seeing how these results hold up in real Parkinson’s patients would be the most crucial next step. Although there is some information available regarding NAD+ precursors in humans—a few small clinical trials have indicated that NR and NMN may improve overall health, mitochondrial function, and inflammation in older adults—there is insufficient data regarding Parkinson’s disease in particular. The majority of research on NAD+ precursors in people has concentrated on metabolic disorders, Alzheimer’s disease, and aging.
Perhaps the most relevant clinical work conducted by Haukeland University Hospital in Bergen, Norway. In 2022, a group led by Charalampos Tzoulis published results of a small clinical trial, revealing that consuming up to 1000 mg of NR in capsules per day provided clinical benefits to a group of 30 Parkinson’s patients. A year later, Tzoulis and his colleagues published new findings from another small study in which they tested the limits of NR supplementation (3000 mg/day) in 20 Parkinson’s patients for four weeks. Although NR treatment was well tolerated and did not result in any moderate or severe adverse events, levodopa therapy overshadowed a clinical benefit that was seen for the patients.
In the end, it is still unknown if NR or other NAD+ precursors, like NMN, can prevent Parkinson’s disease in people. With the Norwegian researchers continuing their research, however, we might soon have a more definitive answer.