Key Points:
- Based on previous twin studies and mathematical modeling, our lifespan is 54% determined by our genetics.
- Previous studies may have underestimated the contribution of genetics by including participants who died of environmental factors not related to intrinsic aging.
A new pre-print (not yet peer-reviewed) paper challenges the current consensus on the genetic contribution to human lifespan. While previous studies estimated heritability at 15-33% (typically 20-25%), with some suggesting it could be as low as 7%, this study demonstrates that when confounding factors are properly addressed, the intrinsic heritability of human lifespan is approximately 50%. Thus, this estimate is more than double previous heritability estimates, suggesting genes play a much larger role in determining lifespan than once thought.
The Problem with Previous Studies
Previous studies on the heritability of human lifespan had two major problems that led to significant underestimation of genetic contribution:
- Failure to account for extrinsic mortality: Previous studies didn’t properly account for deaths from external causes (accidents, infections, homicide, environmental hazards). The focus should be on intrinsic mortality — deaths stemming from processes originating within the body, including genetic mutations, physiological decline, and age-related diseases (e.g., heart disease, dementia, and type 2 diabetes).
- Inconsistent cutoff ages: Previous longevity studies used different minimum age thresholds (ranging from 15-37 years) for inclusion, making it difficult to accurately predict heritability.
These problems were addressed in the new paper, as scientists from the Weizmann Institute of Science in Israel, the Karolinska Institute in Sweden, Westlake University in China, and Leiden University Medical Center in the Netherlands investigated the effect of extrinsic mortality and cutoff age on estimates of heritability.
54% of How Long We Live is determined by Genetics
With the emergence of industrialized society, extrinsic mortality — deaths caused by factors originating from outside the body — has declined. The decline in extrinsic mortality is primarily due to improved sanitation and control of infectious diseases (e.g., HIV, flu, measles). For this reason, the researchers employed a sophisticated approach to isolate and remove the effects of extrinsic mortality and estimate the heritability of intrinsic lifespan.
Using mathematically modeled computer simulations, incorporating data from a Danish twin study and a Swedish twin study, the researchers systematically decreased extrinsic mortality rates. This led to an increase in the correlation between twin pair lifespans, an important factor in determining heritability. To calculate heritability, the researchers used the Falconer formula, which includes how strongly the lifespans of twin pairs are correlated:
Heritability = (r MZ – r DZ)
In this equation, r is the correlation strength, MZ is monozygotic twins, and DZ is dizygotic twins. It follows that reducing extrinsic mortality increases r for both monozygotic and dizygotic twins, which increases heritability.
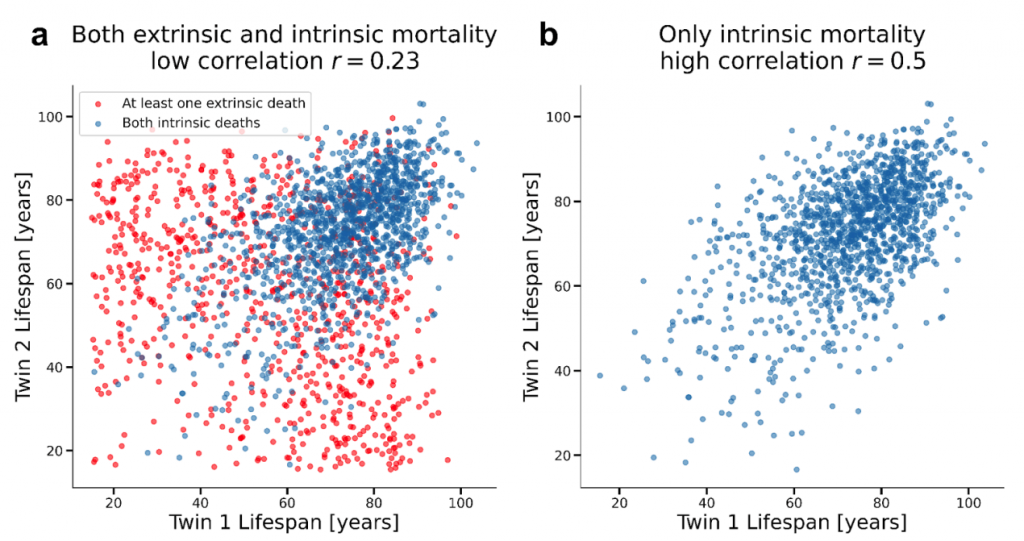
One of the main findings of the study was that removing extrinsic mortality from the Danish and Swedish twin studies consistently resulted in heritability values that plateaued around 50%.
The researchers also ran their simulation on data from a third study called the Swedish Adoption/Twin Study on Aging (SATSA). An advantage of the SATSA is that it includes twins who were raised apart. The simulation showed that removing extrinsic mortality from this cohort resulted in a heritability of lifespan close to 39%. However, if the minimum cutoff age was lowered to 15, the heritability value increased to 53%.
Indeed, the researchers found that, when extrinsic mortality is removed, heritability increases as the minimum cutoff age decreases. For example, the heritability of lifespan estimate is 60% when the cutoff is 15, whereas the heritability estimate is 40% when the cutoff is 50. The authors conclude that a lower cutoff age is preferable because it includes early deaths that inform the variation between individuals.
“We propose defining the heritability of intrinsic lifespan (HIL) as the heritability estimate obtained under zero extrinsic mortality and a cutoff age of 15. We chose age 15 because studies of cause-of-death patterns indicate that intrinsic mortality, driven by biological aging and disease, begins to rise after puberty, typically around age 15. In many historical and modern cohorts, mortality is minimal around this age,” said the authors. “HIL is the genetic contribution to lifespan due to intrinsic biological deterioration, conditional on surviving childhood and reaching reproductive maturity. Using this definition, we find that predicted HIL is consistent across the three twin datasets… …yielding an estimate of [54%].”
Reversing Aging by Altering Genes
A meta-analysis of over 15 million twin pairs showed that most human traits are about 50% heritable, agreeing with the findings that lifespan is about 50% heritable. In that respect, the study challenges the consensus that genetics has only a minor effect on lifespan. The findings have implications for understanding aging mechanisms and informing medicine and public health. If genes explain the majority of lifespan variation, understanding the genetics of longevity can reveal how we can slow aging and deter age-related diseases.
While variations of single genes, such as the SIRT6 gene, are associated with a longer lifespan, lifespan is likely polygenic — determined by many genes. In this case, gene therapy, which at this point can only add or remove single genes, may not be the best option. Until advancements in biomedical technology allow therapeutics to alter polygenic traits like lifespan, we can make lifestyle choices that modulate our extrinsic mortality. Lifestyle choices linked to a longer lifespan absent of chronic diseases include not consuming excess calories, exercising regularly, sleeping 7 to 8 hours per night, and having a purpose.