Key Points:
- Leakage of digestive enzymes from the gut coincides with their accumulation in organs like the heart, lungs, liver, kidney, and brain in old rats.
- These digestive enzymes degrade the organs’ structural proteins as well as extracellular domains of insulin receptors in the brain—which correlates with increased blood sugar.
- Inhibiting the digestive enzyme, trypsin, helps reconstitute the intestinal barrier, restores structural proteins in organ tissues, and reduces blood sugar.
Published in PLoS ONE, Schmid-Schönbein and colleagues from the University of California, San Diego show that digestive enzymes leaked from the gut contribute to organ tissue structural protein (collagen) degradation in old rats. These digestive enzymes, such as trypsin and amylase, also break down insulin receptors in the brain—and possibly other organs—leading to higher levels of blood sugar (glucose), potentially from insulin resistance. Furthermore, inhibiting the enzyme, trypsin, helps refurbish the intestinal barrier, restore organ tissue structural proteins, and reduce blood glucose. These findings may shed light on a key mechanism—digestive enzyme leakage from the gut—contributing to age-related tissue dysfunction.
The pancreas releases digestive enzymes into the intestines after we eat, and these enzymes break down molecules like proteins and fats from food for their digestion. The thing is that these enzymes not only disintegrate biomolecules from food but also others that they come into contact with, such as those found in organ tissues. As Schmid-Schönbein and colleagues’ thinking goes, digestive enzymes released from the pancreas following a meal leak from the gut and enter circulation to thereby degrade tissue in organs like the heart, lungs, and brain—a process dubbed autodigestion. Moreover, as autodigestion progressively worsens with age, not only do tissues and organs deteriorate but, since ensuing tissue repair drives inflammation, autodigestion could also help explain the sterile, low-grade inflammation that develops with advanced age—known as inflammaging.
“This research brings to light that whereas life is only possible with digestion (of the food we eat), there is a price to pay in the form of autodigestion (of one’s own tissue) due to pancreatic digestive enzymes,” said corresponding author of the study, Geert Schmid-Schönbein of the University of California San Diego, in a press release. “Autodigestion is consistent with end-of-life multi-organ failure.”
Reversal of Autodigestion-Induced Organ Deterioration
To test the previously unexplored autodigestion aging mechanism, Schmid-Schönbein and colleagues compared enzyme levels between young and old rats. Interestingly, they found low levels of the digestive enzymes trypsin, elastase, lipase, and amylase in young rat organs, yet these enzymes substantially infiltrated vital organs outside of the pancreas in old rats. What’s more, treating old rats with a trypsin inhibitor for two weeks alleviated the buildup of trypsin in organs like the heart. These findings support the notion that pancreatic digestive enzymes exit from a leaky gut to enter circulation and infiltrate vital organs, which gets progressively worse with age.
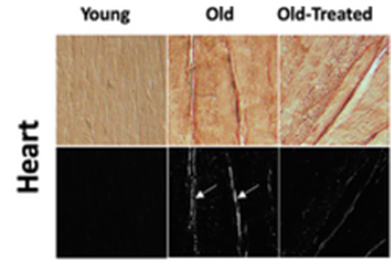
Since digestive enzymes are known to disintegrate most types of biomolecules they come into contact with, the California-based researchers tested whether their elevated levels in organs coincide with organ structural protein—collagen—degradation. Indeed, they found collagen degradation in the lung, liver, heart, and brain, yet inhibiting the digestive enzyme, trypsin, restored collagen in these organs. Thus, these data provide evidence that leaked digestive enzymes from the gut, such as trypsin, indeed facilitate tissue and organ deterioration.
Since many proteins exist on organs, such as insulin receptors, Schmid-Schönbein and colleagues tested their levels in the brains of old rats. As expected, old rats with a leaky gut exhibited a reduced abundance of cell surface insulin receptors in the brain, which correlated with increased blood glucose. Intriguingly, treating these rats with a trypsin inhibitor for two weeks partially restored the density of insulin receptors and lowered blood glucose. These results suggest that circulating digestive enzymes that escape from a leaky gut degrade insulin receptors, which may lower insulin sensitivity and lead to higher levels of blood glucose with age.
To confirm that organ and extracellular receptor degradation stems from progressive autodigestion with age, the researchers examined the intestinal barrier. In that regard, Schmid-Schönbein and colleagues found that the intestinal barrier density declines in old rats. However, treatment with the trypsin inhibitor for two weeks reconstituted the barrier. Hence, this finding supports the notion that a deteriorating intestinal barrier with age may, in fact, leak digestive enzymes to induce the degradation of vital organs outside the pancreas.
Prolonging Periods without Food Intake May Alleviate Autodigestion
The intriguing findings from Schmid-Schönbein may help explain the progression of two hallmarks of aging—the degradation of networks of proteins and other molecules supporting cells (known as the extracellular matrix) as well as inflammation. Along those lines, collagen makes up a key constituent of the extracellular matrix, and tissue degradation initiates tissue repair to drive inflammation. In this way, digestive enzymes from an aged, leaky gut may work to drive these two hallmarks of aging by degrading the extracellular matrix and thus initiate tissue repair to drive inflammation.
What’s more, the researchers hypothesize that their newly developed explanation for how we age, autodigestion, could shed light on why caloric restriction and timed eating patterns extend lifespan in multiple organisms like mice and monkeys. As such, prolonging periods between meals may help reconstitute the intestinal barrier, lowering amounts of digestive enzymes that enter circulation and subsequent damage to tissues and organs. This may minimize organ damage via autodigestion to potentially lower biological age—an assessment of age based on how well cells and tissues function. Furthermore, since the pancreas releases digestive enzymes following food intake, lowering the quantity of food eaten may also alleviate organ damage from autodigestion.
Moreover, alcohol consumption may degrade the intestinal barrier, driving the leakage of digestive enzymes into circulation.
“Ethyl alcohol can dissolve lipid membranes and thus also damage the mucosal barrier,” says Schmid-Schönbein.
Because trypsin inhibition worked to reconstitute the intestinal barrier, restore collagen in vital organs, increase the density of insulin receptors, and lower blood glucose, long-term trypsin inhibition in humans may serve as a strategy to thwart the ravages of aging. When asked about the potential of this strategy, Schmid-Schönbein said he and his research team are working on such a technique. Accordingly, it could be another 15 years or so before a digestive enzyme-inhibiting treatment undergoes development and receives approval. The speed of translating the findings of this study to the application of such a treatment would also depend on whether the strategy yields successful results in human trials.