Key Points:
- Radiation therapy is commonly given to elderly cancer patients due to complications that may arise with chemotherapy or surgery.
- In mice, NMN reduces radiation-induced intestinal damage, including scarring, inflammation, and cell death.
- NMN essentially restores gut bacteria diversity and composition in mice exposed to radiation.
While radiation therapy is highly effective at killing cancer cells, it inevitably causes damage to healthy tissues. When treating abdominal and pelvic cancer, intestinal injury is a typical side effect. This intestinal injury includes irreversible intestinal fibrosis (scarring), which can lead to other complications like mortality. Thus, there is an urgent need for therapies that protect against radiation-induced intestinal fibrosis.
Zhao and colleagues from the Chinese Academy of Medical Sciences and Peking Union Medical College report in the International Journal of Radiation Biology that NMN alleviates radiation-induced intestinal fibrosis by modulating gut microbiota. They also show that NMN reduces radiation-induced inflammation and cell death. Furthermore, NMN largely restores the changes in gut bacteria diversity and composition reshaped by radiation.
NMN Improves Radiation-Induced Intestinal Fibrosis
Zhao and colleagues exposed the abdomens of mice to radiation and immediately administered 300 mg/kg a day of NMN into their drinking water for 12 months. NMN treatment inhibited collagen deposition, the standard measure of fibrosis, in the intestine of the radiation-exposed mice. Since fibrosis is usually accompanied by inflammation, the investigators measured a molecule known to promote fibrosis called TGF-β and found that NMN decreased this inflammatory molecule in colon tissue. Moreover, NMN inhibited the apoptosis (cell death) of colon cells.
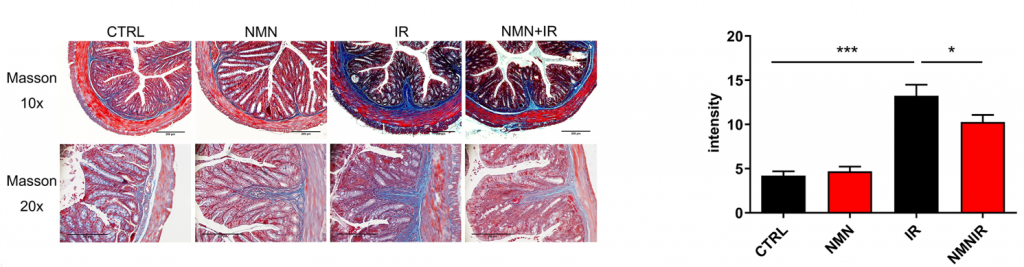
NMN Improves Radiation-Induced Alterations in Gut Bacteria
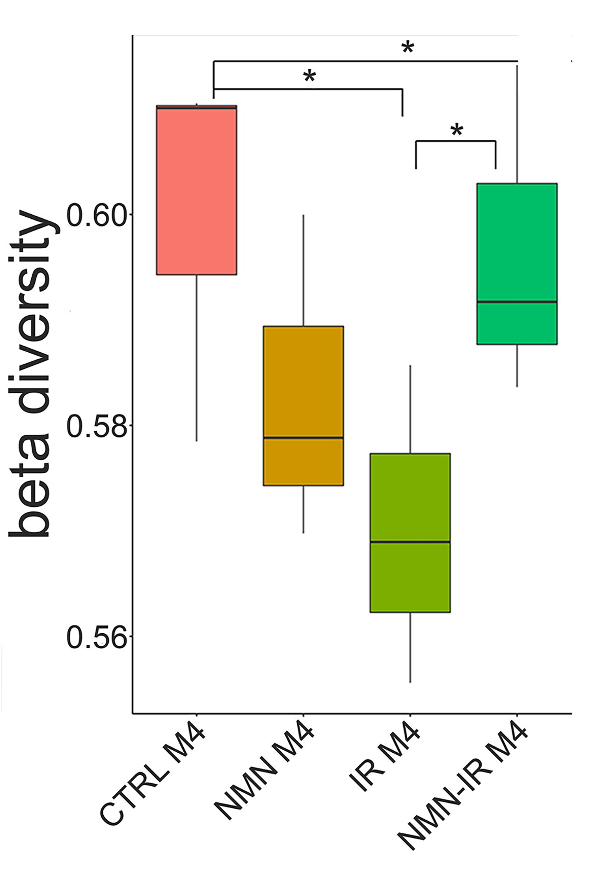
Our gastrointestinal tract is inhabited by gut bacteria capable of modulating our nutrient intake, energy metabolism, and immune function. Radiation has been shown to alter the abundance and diversity of gut bacteria, which could affect intestinal damage. To investigate whether the radioprotective effects of NMN is related to gut bacteria, the feces of mice were collected four months after radiation exposure, and the genes of the bacteria contained within were analyzed.
While radiation exposure led to a decrease in gut bacteria diversity and composition, this diversity and composition was largely recovered by NMN treatment. Furthermore, using predictive analysis, Zhao and colleagues assessed the functional alteration caused by changes in gut bacteria composition. They found that radiation led to functional changes associated with human disease. However, NMN treatment was associated with functional changes in metabolism, which the authors suggest could help repair the intestinal wall.
Boosting NAD+ to Prevent Cancer Therapy Side Effects and Enhance Cancer Immunotherapies
Overall, the findings of Zhao and colleagues suggest that NMN protects against radiation-induced intestinal injury, in part by reducing alterations to gut bacteria composition and function. A similar study has shown that NMN protects the intestinal wall from radiation-induced damage by reducing oxidative stress and activating pro-longevity molecules. These studies point to NMN as a candidate for reducing cancer therapy side effects. Indeed, NMN combined with troxerutin was shown to fully protect the heart from damage caused by the chemotherapy drug doxorubicin.
NMN may also enhance the effects of immunotherapies, a newly established type of cancer therapy that utilizes immune cells to suppress tumor progression. Animal studies have shown that NMN enhances natural killer cell immunotherapy and boosts CAR-T cell immunotherapy. Other NAD+ precursors have also been shown to have similar effects to NMN. The NAD+ precursor NR has been shown to enhance immunotherapies, and NRH prevents tumor regrowth after brain cancer surgery. Thus, boosting NAD+ with its precursors may pair well with traditional cancer treatments.