Key Points:
- Senescent (aged) cells accumulate with age and contribute to multiple age-related diseases.
- Elevating NAD⁺ levels may increase the production of harmful molecules secreted by senescent cells.
- Removing senescent cells may enhance the anti-aging effects of NAD⁺ boosters.
Scientists are beginning to identify the underlying causes of aging. The hope is that targeting these underlying causes, called the hallmarks of aging, can prevent or delay multiple leading causes of death, including diabetes, cancer, heart disease, and Alzheimer’s. Under this new paradigm, death — the inevitable end of life — can be postponed.
Lying at the interface of several hallmarks of aging is nicotinamide adenine dinucleotide (NAD⁺), a small multifunctional molecule involved in a diverse array of cellular processes. As life progresses into its later stages, NAD⁺ is depleted from our cells, including our immune, brain, and muscle cells. For this reason, NAD⁺ precursors like nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) are currently under investigation for potentially alleviating multiple chronic age-related diseases.
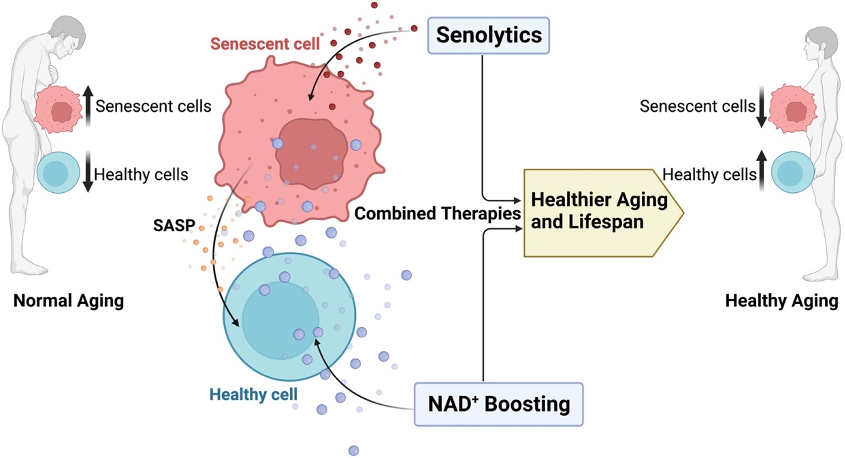
However, simply restoring NAD⁺ levels is not the cure for aging. This is because NAD⁺ repletion not only rejuvenates normal healthy cells, it reinvigorates all cells, even the “bad” ones. Herein lies the complex relationship between NAD⁺ and senescent cells. In the context of aging, senescent cells are the bad cells, implicated in driving the aging process. This is why, like NAD⁺ precursors, scientists hypothesize that senolytics — compounds that selectively destroy senescent cells — could treat multiple diseases.
Considering the anti-aging potential of NAD⁺ boosters and senolytics, Mayo Clinic scientists propose combining the two in the newest volume of Aging Cell. Chini and colleagues review the latest NAD+ and senescent cell research, concluding that the combination of restoring NAD+ and removing senescent cells may be necessary for prolonging the lifespan of humans.
A Short Senescence Story
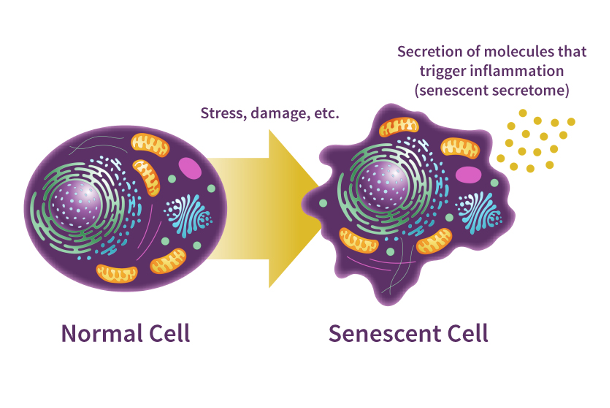
Senescent cells are like inept cells, unable to grow and divide (proliferate) while failing to contribute to the function of the organs they inhabit. To make things worse, they secrete a panel of molecules collectively known as the senescence-associated secretory phenotype (SASP). Primarily via inflammation, SASP molecules from senescent cells slowly degrade the organs and tissues they affect, leading to disease. For example, senescent cells in the brain are linked to neurodegeneration and dementia while senescent cells in muscle are linked to muscle atrophy and weakness.
Furthermore, no healthy cell is safe from a senescent fate. While SASP molecules can spread senescence to neighboring cells, senescence can also be triggered by cellular stressors. One of these cellular stressors is DNA damage, which can lead to genetic mutations. Indeed, the purpose of senescence may be to stop cancer progression by halting the proliferation of mutated cells. This anti-cancer safeguard works well in healthy individuals, as immune cells called natural killer cells can destroy senescent cells. However, as our immune system weakens with age, senescent cells accumulate.
NAD⁺ and Senescence
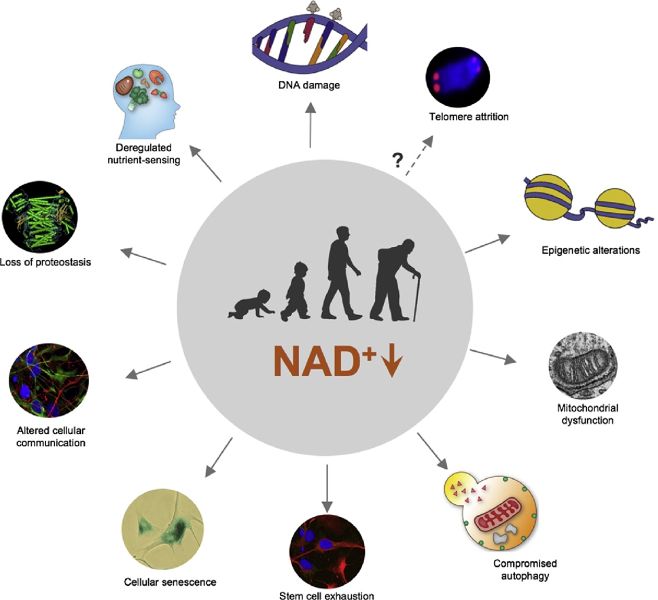
There are 12 recognized hallmarks of aging. Remarkably, low NAD+ levels are associated with at least 9, one of which is cellular senescence. Specifically, low NAD⁺ levels induce DNA damage and mitochondrial dysfunction, which are cellular stressors that trigger senescence. In this way, low NAD⁺ levels promote the accumulation of senescent cells.
However, low NAD⁺ levels may also inhibit the production of SASP molecules. This is because the production of SASP molecules requires an abundance of cellular energy called ATP. ATP is primarily generated by mitochondria, and this generation of energy is mediated by NAD⁺. It follows that, without sufficient concentrations of NAD⁺, mitochondria produce ATP less efficiently, which can inhibit the production of SASP due to a lack of energy. Therefore, boosting NAD⁺ could support the production of SASP molecules by rejuvenating mitochondria and replenishing cellular energy supplies.
Indeed, SASP molecules have been shown to include NAMPT, an NAD⁺-synthesizing enzyme that boosts NAD⁺ levels. Further studies propose that NAMPT supports the production of pro-inflammatory SASP molecules by boosting NAD⁺ levels. This poses a problem, as pro-inflammatory SASP molecules are conducive to cancer progression and NMN has been shown to strengthen pro-inflammatory SASP production.
Adding to the complexity, senescent cells also promote the depletion of NAD⁺ by stimulating the elevation of CD38. CD38 is an NAD⁺-degrading enzyme that accumulates with age and is recruited to organs and tissues by senescent cells. Moreover, SASP molecules can induce CD38 activation in both senescent and normal cells, contributing to the age-related increase in CD38.
Getting rid of senescent cells or SASP molecules has been shown to decrease CD38 and boost NAD⁺ levels. Furthermore, blocking CD38 boosts NAD⁺ levels and increases the lifespan of mice. Therefore, removing senescent cells with senolytics could indirectly lead to a prolonged lifespan by decreasing CD38 and mitigating the degradation of NAD⁺.
Combining Senolytics and NAD+ Precursors to Counter Aging
Upon reviewing the latest age-related NAD⁺ and cellular senescence research (summarized above), Chini and colleagues remark,
“One possibility is that the potential benefits of NAD+ boosting therapy may be offset by the fact that this therapy may not decrease the senescence burden and may even exacerbate the SASP.”
The Mayo Clinic scientists go on to say,
“Understanding the connection between NAD metabolism and senescence during the aging process would provide crucial knowledge to help the development of therapies for improving healthspan and preventing and treating age-related diseases.
Therefore, combining senolytic therapy with NAD boosting may enhance the efficacy and safety of NAD-targeted interventions. Possibly, the combination of these therapies may have added/synergistic benefits in aging, healthspan, and longevity.”
They conclude,
“We believe that understanding the interplay between NAD metabolism and cellular senescence could greatly impact our understanding of the potential therapies for age-related conditions. Thus, exploring the beneficial and adverse effects of combining NAD boosters with senolytic agents is imperative.”