Key Points:
- Treatment with Apre enhances cognitive function in diabetic rats with learning and memory deficits.
- Diabetic rats treated with Apre exhibit fewer degenerating cells in the hippocampus – the brain region for learning and memory – and a decrease in both amyloid beta and tau-positive cells – two hallmarks of Alzheimer’s disease.
- Apre treatment reduces inflammation, oxidative stress, and insulin resistance, all of which drive memory decline.
Alzheimer’s (AD) continues to plague the minds of people around the world, and still, scientists have yet to uncover the cure for this neurodegenerative disease. New evidence suggests that a class of enzymes called phosphodiesterase-4 (PDE4) inhibitors hold anti-inflammatory, neuro-protective, and memory-boosting properties that could potentially be beneficial to AD patients. Unfortunately, most PDE4 inhibitors are known to induce vomiting, and this severe adverse reaction has ultimately impeded their clinical approval. However, one PDE4 inhibitor with the potential to surpass this roadblock is the psoriasis drug apremilast (Apre), which has minimal ties to nausea and vomiting.
In a new study published in the journal International Pharmacology, researchers from Assiut University in Egypt explore the effects of Apre in diabetic rats modeling AD, which was induced by a hight-fat/high-fructose (HF/HFr) diet combined with the chemotherapy drug streptozotocin. Gomma and colleagues found that treating diabetic AD rats with Apre significantly attenuated learning and memory deficits and reduced cell degeneration in the hippocampus. What’s more, the levels of amyloid beta and tau-positive cells were lower following treatment. Notably, Apre treatment also reduced multiple markers of cognitive impairment, including inflammation, oxidative stress, and insulin resistance.
Apre Enhances Learning and Memory
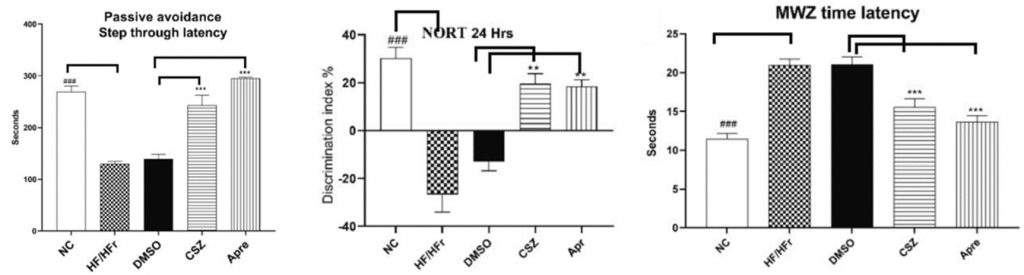
To examine Apre’s role in learning and memory, Gomma and colleagues conducted an array of behavioral tests, namely, the passive avoidance test, novel object recognition test, and Morris water maze test. They also compared Apre’s efficacy to another PDE inhibitor, cilostazol (CSZ).
The passive avoidance test is a fear-motivating learning task where rodents are evaluated on their ability to recall aversive stimuli. Here, rats were placed next to a dark chamber, which, upon entry, induced a foot shock. Rats that exhibited increased latency to enter the dark chamber following the conditioning phase were characterized as rats with superior learning and memory abilities, as those rats were still aware of the negative foot shock. Moreover, the investigators found that the latency to enter the dark chamber was greatest in rats treated with Apre.
The novel object recognition task was utilized to evaluate long-term recognition ability, which drastically declines in people with AD. This test examined the spontaneous tendency of rodents to spend more time exploring a novel object than a familiar one. Gomma and colleagues showed that AD rats treated with Apre explored the novel object significantly longer than untreated rats and CSZ-treated rats, indicating superior recognition.
The Morris water maze test assessed spatial learning, where rodents were trained to find a hidden platform in a pool of water. As expected, rats treated with Apre located the platform much more quickly than their untreated counterparts and CSZ-treated rats. Overall, the findings suggest that Apre is more effective than CSZ and holds cognitive-boosting properties that contribute to enhanced learning and memory.
The investigators proceeded to look at hippocampus tissue following treatment and found that Apre significantly reduced the amount of degenerating cells, indicating a healthier brain. Furthermore, there were lower levels of the toxic proteins tau and amyloid beta, both of which are believed to drive AD, in Apre-treated rats. Given these findings, these results indicate that Apre ameliorates multiple features of AD.
Apre Mitigates Oxidative Stress, Inflammation, and Insulin Resistance
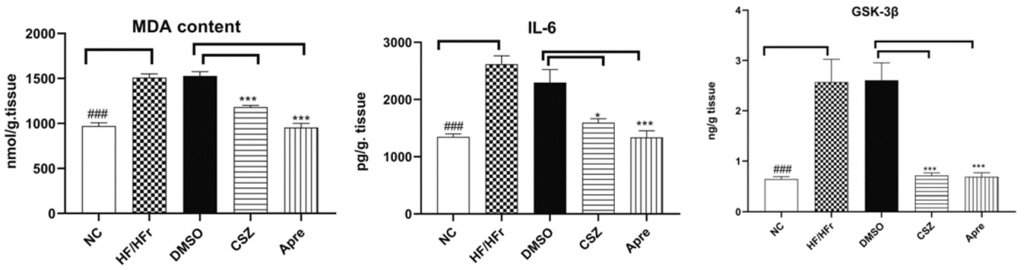
After confirming Apre’s protective effects on cognitive decline, Gomma and colleagues sought to find any potential underlying mechanisms responsible for the observed learning and memory improvements. Accordingly, the investigators examined markers of oxidative stress (MDA) and inflammation (IL-6), as these factors are well-established promoters of memory decline. The results showed that MDA and IL-6 levels were significantly lower in rats treated with Apre than in untreated rats, demonstrating Apre’s profound anti-oxidative and anti-inflammatory properties.
The Egyptian researchers also looked at a marker of insulin resistance (GSK-3β), a common feature of diabetes that increases the risk of AD. The findings showed that Apre-treated rats exhibited drastically less GSK-3β activity than untreated rats. In the final analysis, the data suggest that Apre exerts protective effects on cognition through the regulation of oxidative stress, inflammation, and insulin resistance.
Apre and Aging
In addition to Gomma and colleagues confirming Apre’s potential to mitigate multiple cognitive deficits linked to AD, other researchers have shown that Apre holds senolytic properties, meaning it can attenuate cellular senescence – a critical state where cells no longer grow or divide. Studies have pinpointed senescent cells as a central hallmark of aging, and their accumulation is tied to the progression of multiple deadly diseases, including AD, heart disease, and osteoarthritis. With this in mind, it would be interesting to see if Apre’s senolytic, anti-inflammatory, and antioxidative properties translate to other age-related conditions.