Highlights
- Male mouse survival is significantly enhanced through late-life rapamycin administration regimens.
- Female mouse survival was also improved through continuous rapamycin administration.
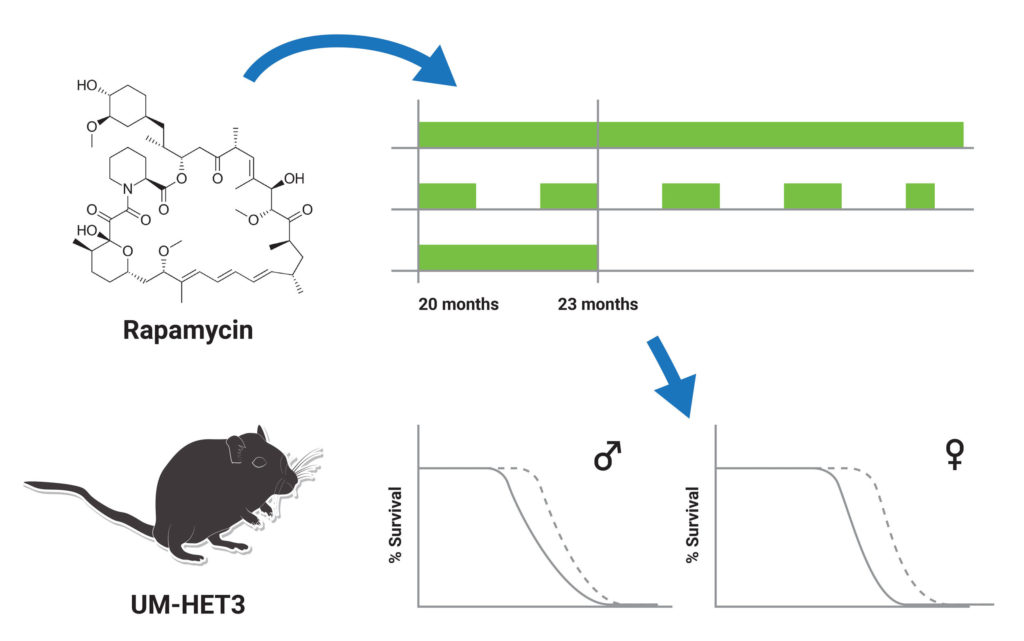
As males and females have different life expectancies, there are likely to be aging variations between them. However, it is still unclear if there is a connection between these variations in aging and responsiveness to treatments that act as anti-aging agents. Furthermore, little is known about sex-specific differences in relation to optimal dosage regimens that maximize the efficacy of age-combatting drugs.
In a published study from the journal Aging Cell, Harrison and colleagues from the Jackson Laboratory in Maine demonstrated how mouse life span improved in a sex-specific manner after administration of late-life rapamycin dosage regimens. In the study, 20-month-old mice were either continuously given rapamycin until the 23-month mark, continuously given rapamycin past the 23-month mark, or given rapamycin every other month until the 23-month mark. In male mice, all three treatments were equally effective. However, continuous exposure past the 23-month mark showed to be the most effective in female mice, but the other two treatments were still effective in extending the lifespan of female mice. These results may help further understand the sex-specific effects of different rapamycin dosage schedules and refine sex-specific therapeutics.
Rapamycin May Improve Lifespan by Suppressing Cell Proliferation
Rapamycin, an immune-dampening molecule, has been utilized by clinicians to treat rare lung diseases (lymphangioleiomyomatosis) and to avert the rejection of human organ transplants. By targeting a cell growth regulator enzyme called the mammalian target of rapamycin (mTOR), rapamycin can suppress the proliferation of cells in mammals. However, the specific effects of rapamycin have yet to be elucidated. Life span has been shown to increase in worms, flies, and yeast upon treatment with rapamycin, and scientists propose the possibility of humans using rapamycin upon aging to achieve similar results and improve overall health.
Late-life Rapamycin Dosing Schedules Produce Sex-Specific Lifespan Enhancements
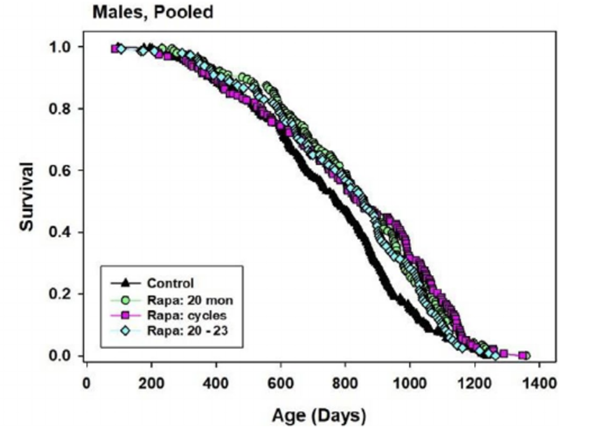
Harrison and colleagues utilized three rapamycin dosing regimens for middle-aged mice to address whether varying rapamycin administration schedules affected survival outcomes. In the first dosing regimen, 20-month-old mice were continuously treated until death with 42 parts per million (the equivalent of 7mg/kg body weight per day) rapamycin concentrations in food until death. This treatment showed significant improvement in male and female lifespan. In the second dosing regimen, 20-month-old mice were treated for three months with the same rapamycin dosage as regimen one; however, this treatment only showed improvements in survival for male mice. For the last dosing regimen, 20-month-old mice were treated every other month with the same rapamycin dosage as the first two regimens. While positive survival effects were observed in both sexes for the last treatment, continuous rapamycin exposure was still the most efficient in extending the lifespan of female mice.
Investigators observed a 9-11% median lifespan increase in males across all three dosing regimens, and these percentages translate to about a 1/3 increase in lifespan extension. In addition, researchers noticed that maximum lifespan increased more in males who were treated with the first and third dosing regimens. However, the second dosing regimen that consisted of continuously giving rapamycin from ages 20 months to 23 months showed no significant increase in extending lifespan. Nevertheless, Harrison and colleagues failed to demonstrate any significant differences in extending median male lifespan through varying dosage regimens.
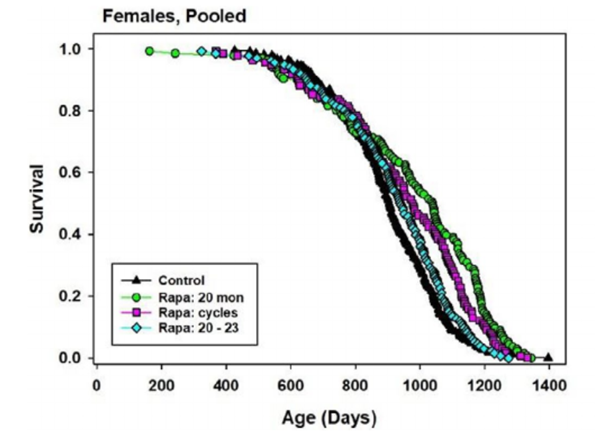
In contrast, survival benefits in females differed based on the treatment protocol recieved. When 20-month-old female mice were continuously treated with rapamycin, median lifespan increased by 15%. For mice treated with rapamycin every other month, median lifespan increased by 8%. Lastly, 20-month-old female mice that were continuously treated with rapamycin for three months showed a 4% increase in median lifespan. Continuous administration of rapamycin in 20-month-old mice demonstrated to be the most effective in females and was also shown to facilitate a 40% increase in female lifespan. In attempts to examine variations in maximum lifespan, investigators concluded that continuous treatment with rapamycin from age 20 months to 23 months did not induce longer lives. However, the other two regimens did promote a longer lifespan.
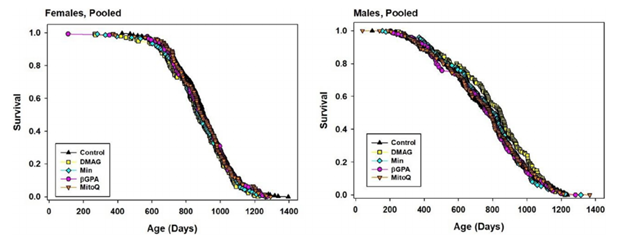
Four Drugs with No Effect on Mouse Lifespan
Harrison and colleagues proceeded with giving mice four other food treatments that have previously shown potential in enhancing longevity. 17-DMAG, a compound that has been shown to protect mouse neurons and reduce inflammation, was used in the first treatment (30 parts per million for six months). The second treatment consisted of treating mice with B-GPA (3300 parts per million for six months), which has been shown to lower blood glucose and potentially enhance longevity. MitoQ, a cell stress-reducing antioxidant, was utilized in the third treatment with mice undergoing 100 parts per million MitoQ for six months. Lastly, the antibiotic minocycline, which has shown potential in extending lifespan in roundworms and flies, was used in the fourth and final treatment (300 parts per million for six months). The obtained results from the four food treatments displayed no significant longevity enhancements. However, formulating other possible treatment concentrations or dosing regimens may help manifest lifespan-enhancing outcomes.
Developing an Optimal Clinical Dosing Strategy
The investigators of this study have yet to elucidate why varying dosing regimens have a different effect on male and female survival in mice. “It is possible, in addition, that some of the rapamycin effects involve alteration in microbial populations within the GI tract, which could show variable time courses of drug-induced reconfiguration and recovery,” said the authors.
Careful refinement and adjustment of these treatments may be necessary to develop optimal sex-specific rapamycin clinical strategies that induce minimal side effects. “Developing clinical strategies for optimal benefit in long-term treatment, with minimal side effects may require careful stepwise refinement and adjustment for sex-specific effects,” concluded the authors. To address if human longevity can improve with rapamycin treatment and whether differences in efficacy are sex-specific, clinical trials must still be conducted.