Repetitive DNA strands called telomeres stop chromosome ends from fraying or sticking to each other, similar to plastic tips at the ends of shoelaces. These protective ends also ensure DNA gets copied correctly during cell replication. The length of telomeres is closely linked to aging, and when telomere length gets whittled down, cells age faster. Not only that but there’s a complex set of conditions called telomere biology disorders characterized by dysfunctional telomere maintenance. One of these disorders is a rare, inherited disease called dyskeratosis congenita, a bone marrow failure syndrome, with no means for effective treatment.
A research team from the National Institute on Aging published in The EMBO Journal showing that cells from patients with dyskeratosis congenita and mice that have short telomere display lower levels of the vital molecule nicotinamide adenine dinucleotide (NAD+). They show that replenishing NAD+ levels with nicotinamide riboside (NR) ameliorates defects in cells from patients with dyskeratosis congenita and mice with short telomeres. “These findings reveal a direct, underlying role of NAD+ dysregulation when telomeres are short and underscore its relevance to the pathophysiology and interventions of human telomere-driven diseases,” said the researchers in their publication.
Telomere deterioration and dysfunction of the cell’s powerhouse, the mitochondria, are hallmarks of biological aging and drive telomere biology disorders. Mitochondrial impairment and defective DNA damage responses accelerate telomere dysfunction and substantially reduce the lifespan where cells can replicate in dyskeratosis congenita through a phenomenon called cellular senescence. Also, declining NAD+ levels has emerged recently as a feature of aging in humans and animals. This happens in part because of the increased levels of an NAD+-consuming enzyme called CD38, which distorts the activities of other enzymes that depend on NAD+ in aged mice.
Liu and colleagues found in their study that cells from dyskeratosis congenita patients and mice with critically short telomeres have lower levels of NAD+, causing enzyme-related signaling networks defects to impair mitochondrial health. Along these lines, they show that when CD38 consumes NAD+, it reduces NAD+ availability to enzymes called PARPs and SIRTs that function in telomere and mitochondrial maintenance.
To gain a better understanding of how elevated CD38 levels contribute to declining NAD+ during aging, Liu and colleagues examined CD38 levels in dyskeratosis congenita cells. They found that CD38 levels were higher in dyskeratosis congenita cells, but this elevation was reduced when the DNA damage response to shortened telomeres was blocked. These findings support the idea that the DNA damage response induced by telomere dysfunction results in elevated activity of CD38, which drives imbalanced NAD+ levels to limit NAD+ availability for PARP and SIRT enzyme activities.
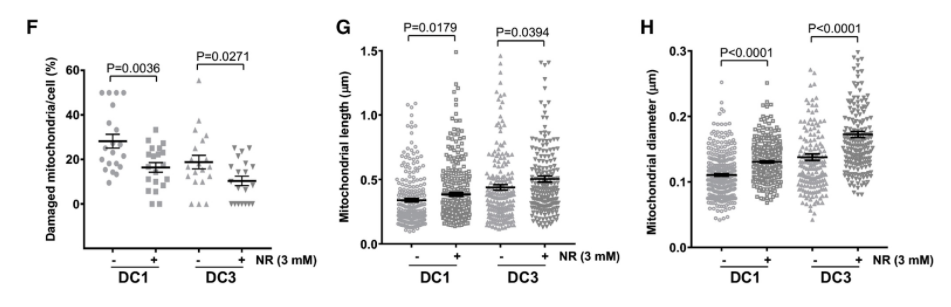
When Liu and colleagues added an NAD+ precursor called NR or a CD38 activity inhibitor in dyskeratosis congenita cells, NAD+ levels were restored and the cellular effects of telomere dysfunction in dyskeratosis congenita patient cells were improved. NR supplementation and CD38 inhibition also restored PARP and SIRT activities, which attenuated telomere DNA damage, mitochondrial impairment, and senescence onset in dyskeratosis congenita cells.
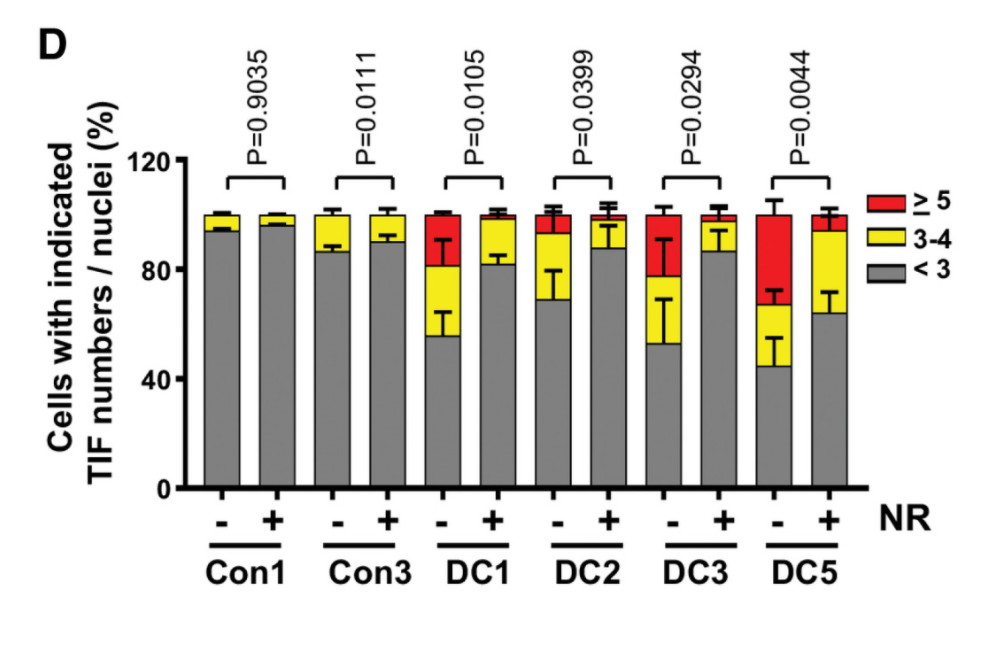
The study’s results support the notion that telomere erosion affects NAD+ levels and related biological pathways, and thus contributes to telomere and mitochondrial abnormalities along with cellular senescence. “Our findings indicate that critically short telomeres and telomere dysfunction leads to loss of NAD+ homeostasis and establish a new linkage between dysfunctional telomeres and mitochondria that contributes to the deleterious consequences of telomere dysfunction,” said the investigators in the article.
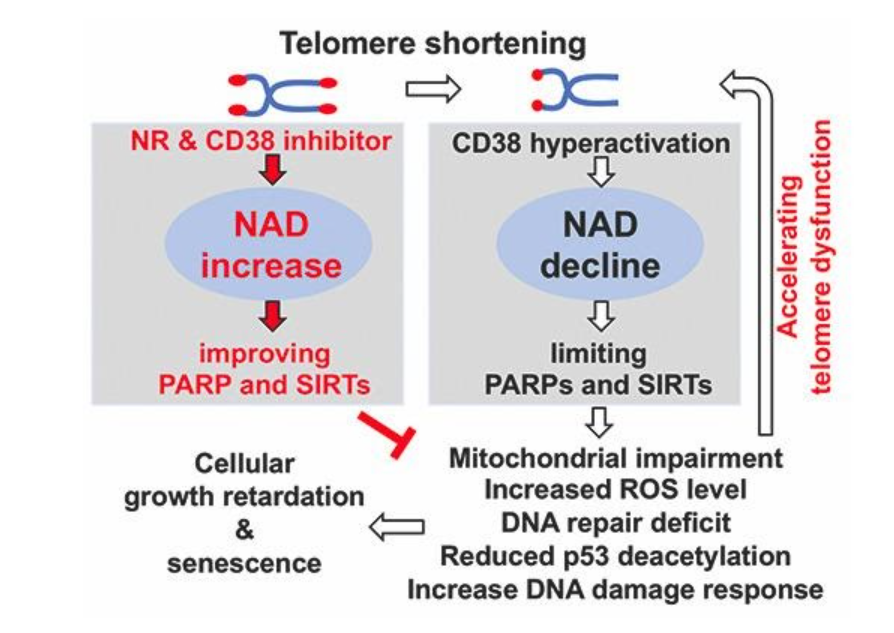
The study’s findings also establish how dysfunctional telomeres contribute to other hallmarks of aging and telomere biology diseases and provide insight for therapeutic intervention development in telomere biology disorders. “The NAD+ mechanism-based intervention strategies developed in this study may serve as a stepping stone for the future treatment of human telomere biology disorders such as DC,” concluded the investigators in the article.