Key Points:
- People with Alzheimer’s disease do not produce enough of a chemical called indole-3-acetic acid (IAA) in the cerebrospinal fluid that surrounds and protects the brain and spinal cord.
- When Alzheimer’s disease mice were fed gold nanoparticles, IAA levels in the body increased and reduced Alzheimer ’s-related symptoms.
Alzheimer’s disease is a progressive neurological disorder characterized by memory loss, cognitive decline, and behavioral changes due to the degeneration and death of brain cells. These changes in the brain are thought to be caused by the buildup of abnormal protein in the brain, causing damage and eventually killing brain cells.
In this study, scientists wanted to see if small molecules engineered in a lab and made with gold could be used to treat Alzheimer’s disease. These small molecules are referred to as gold nanoparticles (Au-NPs). When Au-NPs were fed to mice with Alzheiner’s disease they improved brain function and stopped harmful protein buildup in the brain.
Researchers analyzed the mice to see how exactly these nanoparticles were helping. They found that the gold nanoparticles were altering the population of bacteria living in the colon, also called the gut microbiome. It was also found that the concentration of certain molecules produced by the microbiome had also changed. Alzheimer’s mice treated with gold nanoparticles had more indole-3-acetic acid (IAA) in their bodies compared to the Alzheimer’s mice that did not receive treatment. This is interesting because IAA levels are lower in the blood and brain fluid of humans with Alzheimer’s disease.
Then, to test whether the increase in IAA was responsible for the benefits in the Alzheimer’s mice, researchers fed IAA to Alzheimer’s mice. This also led to improvements in cognitive behavior. This research, published in Nature Aging, provides a promising therapeutic target for the amelioration of neuroinflammation and the treatment of neurodegenerative diseases.
Finding ways to affect the brain by targeting the gut
Research over the past few decades has shown that the gut and brain are intimately connected. Indeed, the gut has the most nerve connections out of any organ in the human body, and many studies have provided data showing that the gut environment can change the nervous system. Accordingly, several neurodegenerative diseases are linked to changes in the abundance and/or diversity of bacteria in the intestines– also called the gut microbiome. The individual composition of gut microbiota may affect how the disease spreads through the gut-brain axis to affect the brain. This is why researchers are now looking into how to treat neurodegenerative diseases through the gut-brain axis, which changes metabolic activity, the immune system, and the way sensory neurons talk to each other.
Some interventions, like changing your diet and taking probiotics can change the diversity and make the gut microbiome more balanced. However, using new, experimental molecules for therapeutic purposes is difficult because we do not know how well they work or if they are safe. Important factors, like the acidic conditions in the stomach (which can break down your molecules) and low permeability of the intestinal wall (preventing these molecules from being absorbed), can limit the effectiveness of this strategy. Therefore, the development of technologies that can overcome these challenges has become a major area of research.
Gold nanoparticles improve Azheimer’s mice by altering gut microbiota
In this article, researchers from Jiangnan University investigated the contribution of gold nanoparticles to the improvement of Alzheimer’s disease through their interaction with the gut microbiota. Gold nanoparticles have shown how they change the gut microbiota and metabolites by changing the activity of microbial genes. Gold nanoparticles were given to mice for 30, 60, and 90 days. This treatment reduced the amount of time it took the Alzheimer’s mice to solve a spatial memory task, suggesting that the gold nanoparticles improve cognitive abilities in neurodegenerative settings.
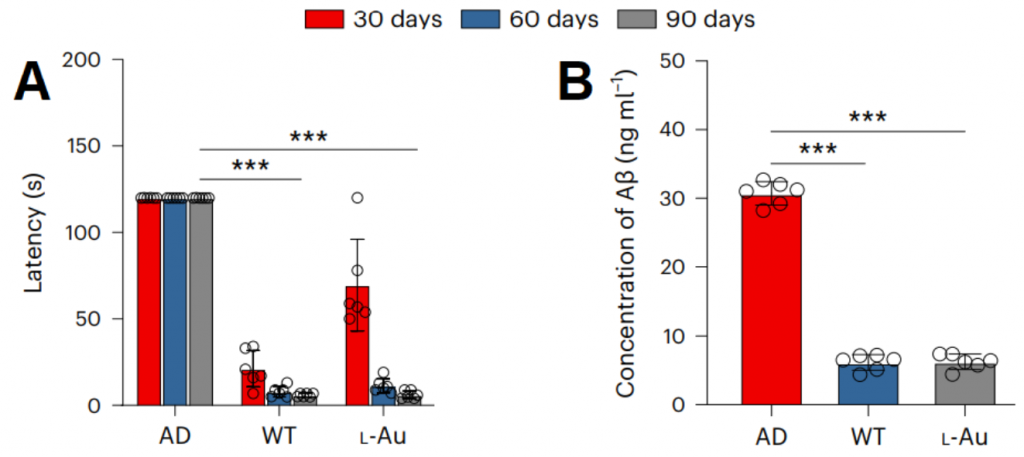
The researchers then discovered that the gold nanoparticles change the make-up of the gut microbiome—the community of trillions of microbes that live in our digestive tracts—and the chemicals that are linked to it. In particular, the gold nanoparticles increased the levels of a gut metabolite called IAA. When IAA was given directly to the Alzheimer’s mice, the researchers observed lowered neuroinflammatory signaling, improved cognitive function, and slowed down disease processes.
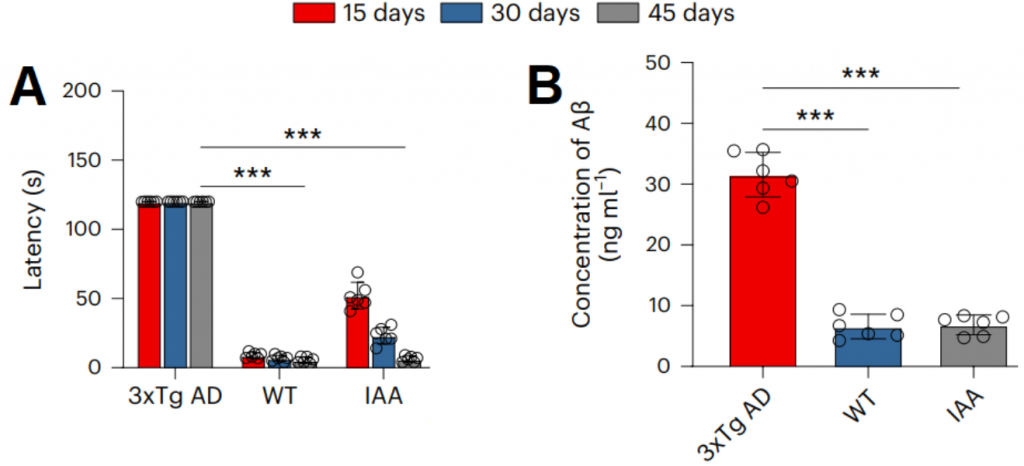
To understand why increased levels of IAA had beneficial effects on Alzheimer’s mice, the researchers looked at the levels of IAA in mice and patients with the neurodegenerative disease. We found that in this study, the amounts of IAA in the blood serum and cerebrospinal fluid (CSF) of mice with Alzheimer’s disease were 91.1% and 80.5% lower than those in “normal” mice. Consumption of the gold nanoparticle by Alzheimer’s mice increased the amount of IAA in their blood serum and cerebrospinal fluid (CSF) by 13.8 and 5.1 times, respectively. Notably, clinical studies on humans showed that IAA levels were much lower in the serum and CSF of people with Alzheimer’s than in healthy humans, which was similar to what was seen in mice. This suggests that the metabolite IAA improves the cognitive abilities and disease characteristics of Alzheimer’s mice.
The researchers say that this finding of how gold nanoparticles can change the gut microbiome opens the door for using this technology to affect the gut-brain axis for a wide range of medical needs.