Several age-related studies seek to understand how gene regulation is affected by methyl groups, which are compounds known to attach to DNA via a process called methylation. Decreases in gene activation are associated with increases in methylation. As humans age, methylation patterns diverge, and cells end up reading incorrect genes, promoting age-related diseases and malfunctional cells. By investigating how age affects patterns in DNA methylation, researchers have been able to better anticipate lifespan in both humans and animals and understand health-related issues that may arise in the future.
In a study published in the journal Nature, Sinclair and colleagues from Harvard Medical School aimed to restore vision in aged mice and mice with glaucoma by reversing the patterns of methylation in the mice’s eye cells. Investigators succeeded in reversing the biological age of cells by promoting DNA methylation patterns seen at youth in the eye cells (retinal ganglion cells) of the tested mice.
“Our study demonstrates that it’s possible to safely reverse the age of complex tissues such as the retina and restore its youthful biological function,” said senior contributor to the study David Sinclair in a press release.
Patterns in methylation are modified upon aging, and it is evident that genes contain more significant amounts of methyl groups as time passes. These findings assisted scientists in formulating DNA methylation clocks capable of predicting a person’s biological age.
Investigators utilized a harmful virus called an adeno-associated virus (AAV) to implement the following three youth-restoring genes into the retinas of mice: Sox2, Klf4, and Oct4. This process allowed the research team to target the eye cells of the aged and injured mice and reverse their DNA methylation clocks. Evidence shows that activation of the three youth-restoring genes generally occurs during embryonic development. In addition, scientists classify these genes as Yamanaka factors.
Investigators successfully observed the regeneration of impaired axons, parts of nerves that transmit signals to other cells, after utilizing AAV to implement the three mentioned youth-promoting genes in the eye cells of young and aged mice. Sinclair and colleagues used forceps to damage the optic nerve, which is comprised of a bundle of axons from eye cells that are directed towards the brain, and then evaluated the impaired axons in mice that obtained Oct4, Sox2, and Klf4 via AAV. Results showed that upon delivery of these three genes to young and aged mice, the structural integrity of axons strengthened, and axons were regenerated.
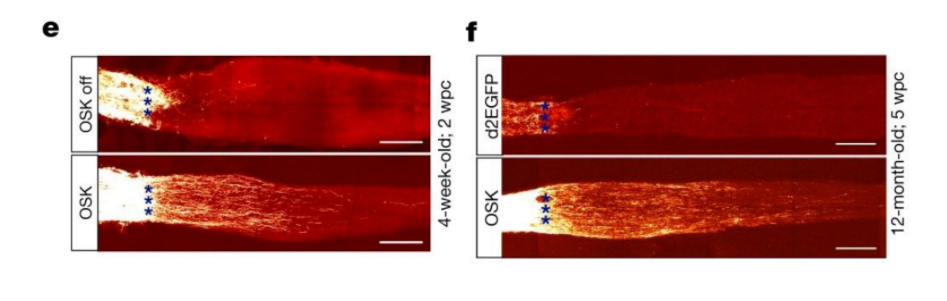
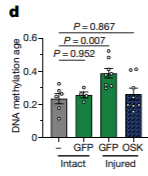
Since patterns in DNA methylation can be reversed by using Yamanaka factors, investigators wanted to test if delivering these genes could help stimulate axon regeneration by reversing the methylation patterns associated with aging. They found that eye cells displayed accelerated DNA methylation age four days after damage, but administration of Oct4, Klf4, and Sox2 dialed back these DNA methylation alterations. In addition, diminished levels of TET1 and TET2, enzymes that reduce DNA methylation on genes, nullified the benefits of gene delivery on the regeneration of axons. These results suggested that utilizing Oct4, Klf4, and Sox2 delivery to restore DNA methylation patterns seen at youth was necessary for regenerating axons.
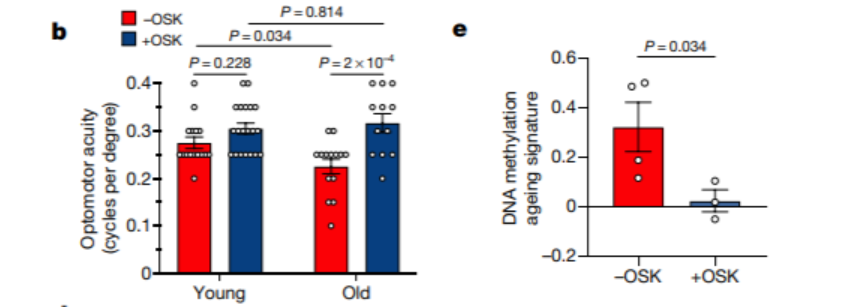
Sinclair and colleagues then succeeded in recovering the vision of mice with glaucoma after the mice received the three genes. They also showed that delivery of the three genes via AAV restored half of the visual acuity that perished from escalated eye pressure. Further investigations showed that delivery of Oct4, Klf4, and Sox2 to old-aged mice facilitated vision recovery that was comparable to the results seen in mice with glaucoma.
“Regaining visual function after the injury occurred has rarely been demonstrated by scientists,” said Meredith Gregory-Ksander, an assistant professor of ophthalmology and co-author on the study in a press release. “This new approach, which successfully reverses multiple causes of vision loss in mice without the need for a retinal transplant, represents a new treatment modality in regenerative medicine.”
This study successfully demonstrated that vision could be restored in aged mice and mice with glaucoma upon delivery of Yamanaka factor genes. Furthermore, investigators showed that improved vision could only be achieved by restoring youthful DNA methylation patterns.
“What this tells us is the clock doesn’t just represent time – it is time,” said David Sinclair in the press release. “If you wind the hands of the clock back, time also goes backward.” Prior to conducting the discussed procedures on humans, similar results must be reproduced in other studies; however, the proposed results suggest that this kind of procedure has the potential to restore vision in humans and repair tissue in older adults.