Key Points:
- Human cells successfully repopulate a rabbit heart that has been decellularized – cleared of all (rabbit) cells.
- When blood is passed through the heart, it visibly pumps.
- The repopulated heart responds to medications that affect heart rate.
Heart attacks can be deadly or cause irreparable damage, especially with advanced age. This is due to age-related deficits in our body’s ability to regenerate heart tissue. However, this regeneration deficit may no longer be an issue if we can grow new hearts in a lab. Now, the results from a new study out of the Texas Heart Institute suggest that we may have found a way to do just that.
In the study, published in Acta Biomaterialia, researchers repopulated a decellularized rabbit heart with various cells generated from human-induced pluripotent stem cells (iPSCs) – skin cells that can be reprogrammed to become different cell types. After 60 days, the heart wall grew to full thickness with the organization and cellular composition of a native heart. Additionally, the heart was shown to visibly pump and respond to chronotropic medications – medications that affect heart rate.
“These results demonstrate the ability to tissue engineer a vascularized, full-thickness [left ventricle] wall with an unparalleled level of …organization and multicellular composition,” the investigators wrote.
Functional Left Ventricle Grown from Scaffolding
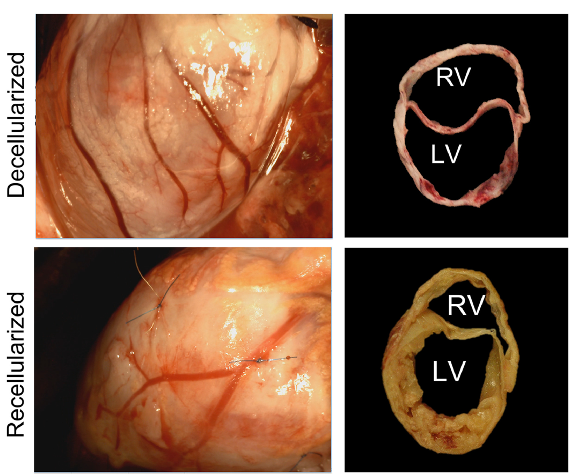
To set up a scaffold, Hochman-Mendez and colleagues decellularized the rabbit’s left ventricle – the part of the heart that pumps blood to the rest of the body. The researchers treated the scaffold with human endothelial cells – the cells that line blood vessels – and added cardiac cells two weeks later. After 60 days, blood vessel networks formed, including arteries – the blood vessels that contain smooth muscle, which allow them to dilate and constrict.
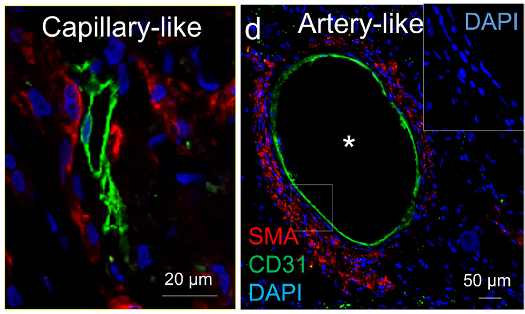
In addition to vascularization by day 60, Hochman-Mendez and colleagues found that the heart tissue recovered 92.6% of its thickness. The recellularized ventricles also showed spontaneous electrical activity, with visible beating. Moreover, the ventricles responded appropriately to medications that affect heart rate. Additionally, by connecting the ventricles to the blood supply of pigs, the researchers showed that blood could flow through the ventricle without clotting, suggesting it should be able to pump blood throughout the body.
Ageless, Artificial, and Animal Hearts
Doris Taylor, the senior author of this study, has been in the heart regeneration business for over two decades. Taylor started with grafting muscle cells into injured hearts and watching them regenerate. However, clinical trials (such as MARVEL and MILES) testing these grafts have shown mixed results with increased risk of irregular heart rates (arrthymias) at higher doses of cells.
Other research has shown the possibility of using animal organs as replacement parts. Pig valves are already used in the clinical setting to replace faulty heart valves in humans, and a recent pig whole heart transplant into a human showed the possibility of using animal organs for patients, but the risk of rejection even with immune suppression is a very real concern. Using only the animal’s heart scaffolding may be a way to alleviate some of the rejection risk.
This study shows that regenerating hearts – or transplanting recellularized hearts – may be a very real future possibility. Hochman-Mendez and colleagues do point out that many more leaps need to be made before a whole heart can be recellularized and regenerated, as this study specifically looks only at the left ventricle.