Key Points:
- Navitoclax increases the median and maximum lifespan of “Titan” mice — a mouse strain selectively bred for high body mass.
- Navitoclax reduces senescent cells — cells that accumulate with aging and contribute to chronic diseases — in the liver.
- Body weight is reduced by Navitoclax treatment towards the end of Titan mouse lifespan.
When our cells are damaged, they become senescent cells, which resist death but can be removed by our immune system. However, with age, our immune system weakens and senescent cells accumulate. Furthermore, like aging, obesity impairs the immune system and induces cell senescence. It follows that senolytics — compounds that destroy senescent cells — could treat obesity-associated aging.
Now, researchers from Germany report in a pre-print (not yet peer-reviewed) from BioRxiv that the senolytic Navitoclax prolongs the lifespan of obese Titan mice. Gille and colleagues also show that Navitoclax reduces senescent cells in the liver and reduces body weight. These findings suggest that senescent cells accelerate the aging of obese mice and removing senescent cells counters this aging.
Senolytic Counters Obesity-Induced Accelerated Aging
To study the effects of senolytics on aging, Gille and colleagues experimented with Titan mice because of their naturally short lifespan. Additionally, unlike other mouse models of aging, Titan mice are not genetically modified and do not require age-triggering compounds. Navitoclax, which inhibits the ability of senescent cells to resist death, was fed to the Titan mice in cycles (5 days on and 16 days off, 5 times) for senolytic treatment.
The researchers found that, compared to the mice fed a placebo, the mice fed Navitoclax lived 16.7% longer median lifespans — the age at which 50% of mice are still alive. Furthermore, the maximum lifespan of the Navitoclax-treated mice was also extended. These findings suggest that clearing senescent cells can prolong the lifespan of obese mice.
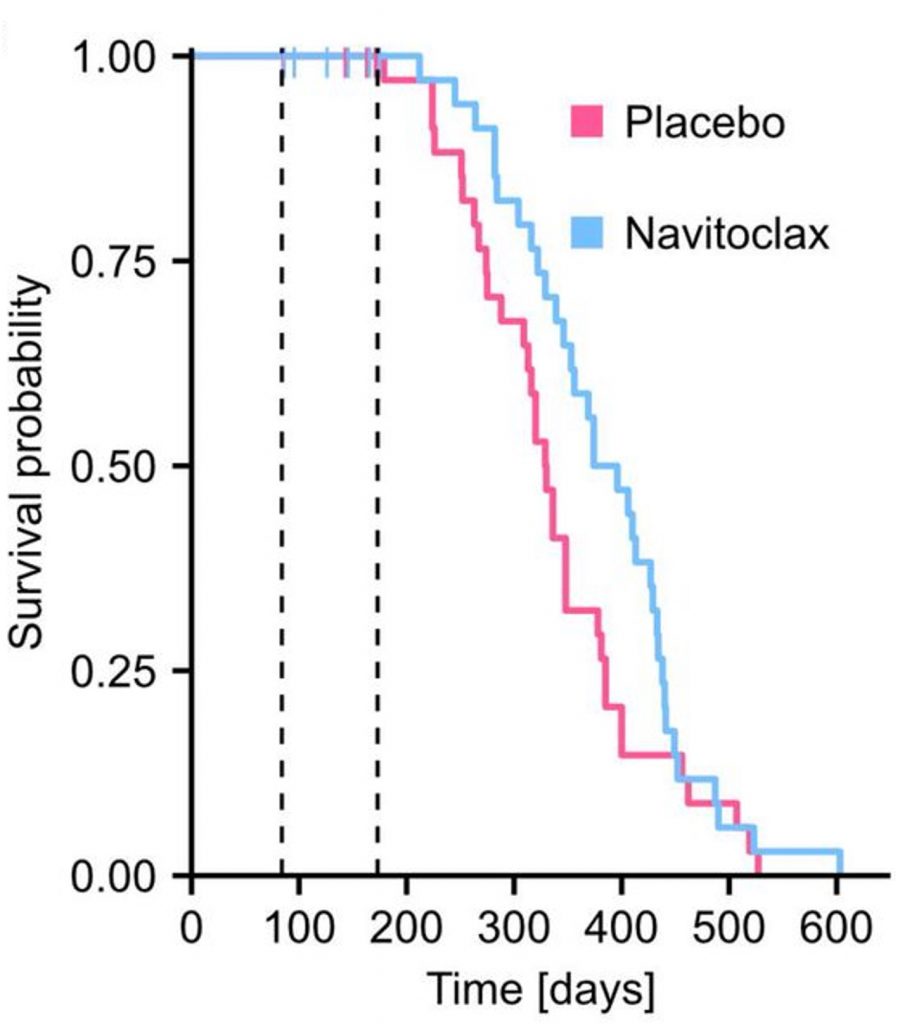
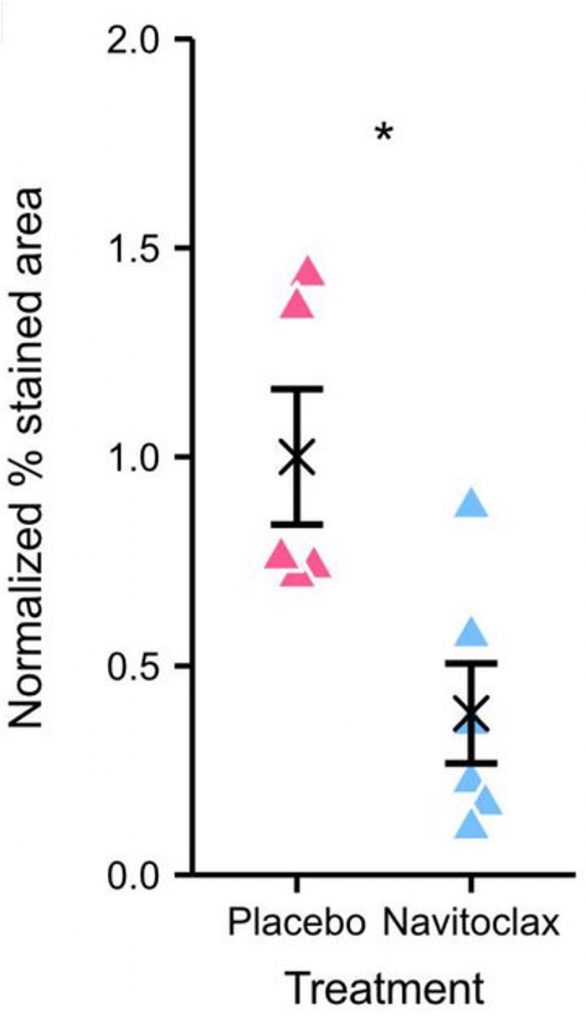
To confirm that Navitoclax reduces senescent cells, Gille and colleagues measured a common senescent cell marker called senescence-associated beta-galactosidase (SA-β-gal) from the livers of obese Titan mice. Compared to the placebo group, the Navitoclax group had greatly reduced SA-β-gal levels, suggesting that Navitoclax removes senescent cells from the liver and potentially other organs.
To determine if Navitoclax leads to weight loss, Gille and colleagues measured the body weight of the Titan mice over a lifetime. The researchers found that Navitoclax-treated mice had less body weight than untreated mice later in life. These findings suggest that senolytic treatment can reduce the weight of obese mice, which could potentially contribute to a prolonged lifespan.
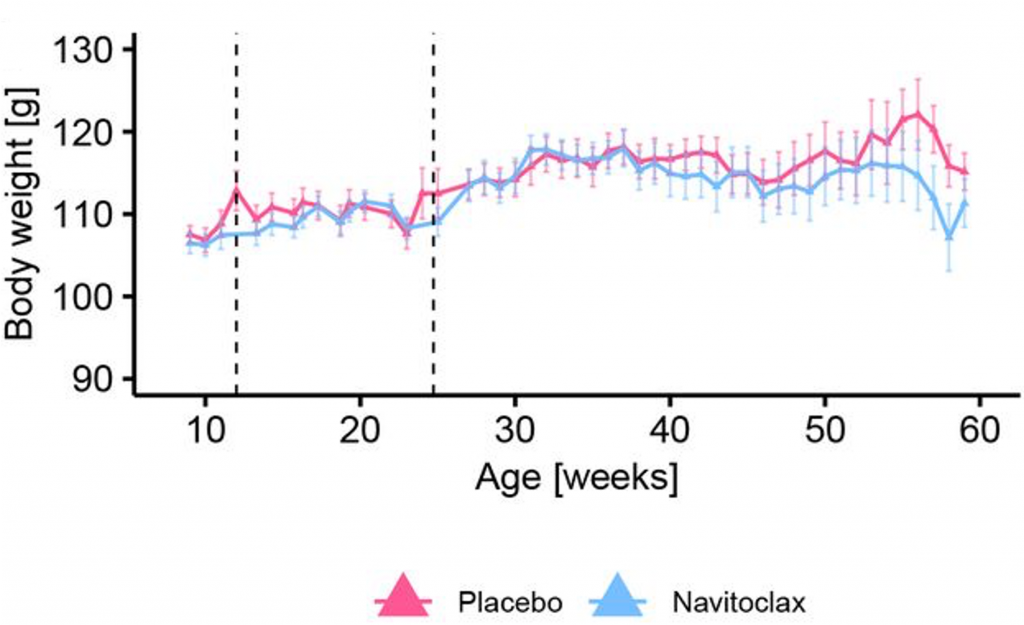
Overall, the findings of Gille and colleagues suggest that the senolytic Navitoclax prolongs the lifespan of obese mice by reducing senescent cells. While further investigation is needed, it is possible that weight loss contributes to Titan mouse life extension.
Other Senolytics that Extend Mouse Lifespan
Navitoclax, also known as ABT-263 is an anti-cancer pharmaceutical repurposed as a senolytic. As a senolytic, it has been shown to rejuvenate human skin, improve age-related memory loss in mice, counter arthritis in humans, and prevent COVID-19 pathology in mice. Now that Navitoclax has been shown to increase the lifespan of Titan mice, the next step is to determine if it can extend the lifespan of non-obese healthy mice.
Only two senolytics have previously been shown to increase the lifespan of healthy naturally aged mice. The senolytic combination dasatinib and quercetin (D+Q) has been shown to extend mouse lifespan by 6%. Additionally, the flavonoid fisetin has been shown to extend mouse lifespan by 10%. However, attempts to replicate the results for fisetin failed. Overall, it seems that senolytics have anti-aging properties, but whether or not they can reliably extend rodent lifespan is unclear.