Key Points:
- Senescent cells — an aging-linked state of permanent cell cycle arrest — from various human tissues reduce levels of the anti-aging protein α-klotho.
- Senolytics — compounds targeting and removing senescent cells — boost α-klotho levels in aged, obese, and senescent cell-transplanted mice.
- Urinary α-Klotho may be a valuable test for following treatments in senolytic clinical trials.
Among the myriad of studied anti-aging molecules, one that has consistently stayed hot is the longevity-linked molecule α-klotho. With extensive studies confirming its neuroprotective effects and ability to increase lifespan in diverse animal models, researchers believe that α-klotho therapies can transform our approach to thwarting age-related cognitive impairment and premature aging. Several companies are even being built around using or activating α-klotho as a therapeutic. However, scientists have yet to determine the optimal modality of increasing cellular α-klotho levels.
As reported in the journal eBioMedicine, Zhu and colleagues show that clearing senescent cells restores α-Klotho, potentially opening a novel, translationally-feasible avenue for developing orally-active small molecule, α-Klotho-enhancing clinical interventions. Selectively removing senescent cells genetically or pharmacologically increased α-Klotho in urine, kidney, and brain of mice with increased senescent cell burden, including naturally-aged, diet-induced obese, or senescent cell-transplanted mice. The senolytic dasatinib and quercetin increased α-Klotho in the urine of patients with idiopathic pulmonary fibrosis (IPF), a disease linked to cellular senescence.
The study’s findings suggest that directly targeting senescent cells with senolytics restores α-klotho levels and potentially protects against age-related diseases linked to α-klotho. “We show that there is an avenue for an orally active, small-molecule approach to increase this beneficial protein and also to amplify the action of senolytic drugs,” says James Kirkland, M.D., Ph.D., a Mayo Clinic internist and senior author of the study. Furthermore, urinary α-Klotho may be a useful test for testing the effectiveness of senolytics in clinical trials on aging and age-related diseases.
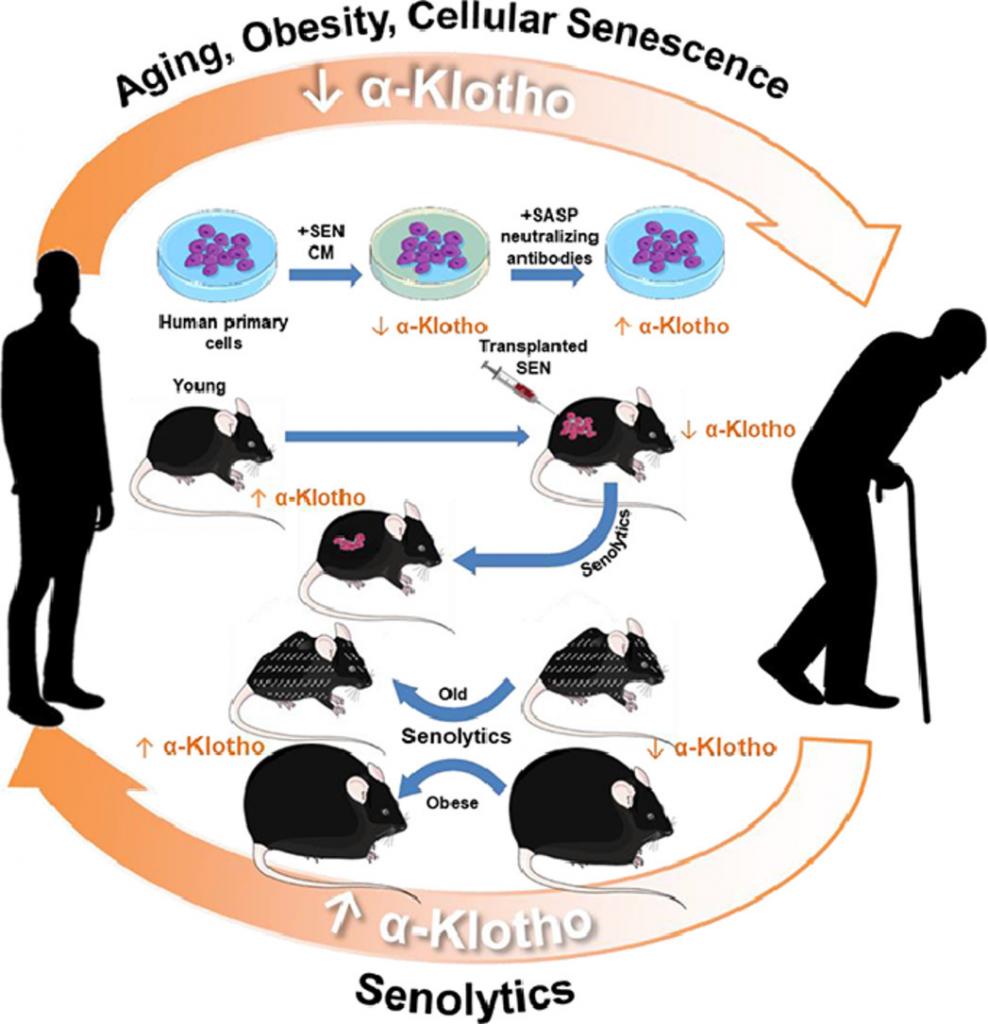
(Zhu et al., 2022 | eBioMedicine) Orally-active, clinically-translatable senolytics restore α-Klotho in mice and humans. α-Klotho and cellular senescence are inversely related and causally linked to the genesis and progression of age-related diseases. Targeting senescent cells increases the geroprotective factor α-Klotho, potentially amplifying beneficial effects of senolytic drugs. This study also opens a novel, translationally-feasible avenue for developing orally-active small molecules to increase α-Klotho, which may also be a useful biomarker for senescent cell burden or senolytic drug activity in clinical trials.
Senescence and Senolytics
One of the hallmarks of aging and the development of age-related diseases is senescent cell accumulation, which progressively increases with age. The mere presence of senescent cells can promote the synthesis of harmful compounds called senescence-associated secretory phenotypes (SASPs), which, when emitted, cause a domino effect to induce more senescent cells. Interestingly, senescent cells are critical for certain biological processes like embryonic development and wound healing. Be that as it may, the extensive documented detrimental effects of senescent cells have led scientists to hone in on senolytic treatments. While future studies are needed to determine the safety and efficacy of senolytics in humans, preliminary findings in various model organisms suggest that senolytic treatments can be critical players in extending lifespan.
Senescent Cells Lower α-klotho Levels
Zhu and colleagues first tested if direct exposure to senescent cells altered α-klotho levels in cells from blood vessels, kidneys, and the brain. To do so, investigators placed these non-senescent human cells in media that contained senescent cells from the same human cell types. Following exposure, α-klotho levels significantly decreased in all human cell types. However, incorporating neutralizing antibodies — proteins that defend cells from various harmful substances — to the media successfully rescued fallen α-klotho levels. These initial findings suggest that SASPs partially contribute to the elimination of α-klotho in non-senescent human cells.
To further clarify whether senescent cells hinder elevated α-klotho levels, the investigators directly transplanted young mice with small concentrations of non-senescent or senescent fat-producing progenitor cells, which specialize in energy storage. Compared to untreated young mice or those treated with non-senescent cells, those treated with senescent cells displayed much lower levels of α-klotho, confirming that senescent cells play a role in modulating α-klotho levels.
Senolytics Boost α-klotho in Mice
Next, Zhu and colleagues analyzed whether treatment with senolytic compounds, specifically the combo of dasatinib plus quercetin or only fisetin, could restore urinary α-Klotho levels in various models senescence: aged mice, young obese mice, and young senescent cell-transplanted mice. In aged and young senescent cell-transplanted mice, both senolytic treatments drastically improved urinary α-klotho levels, with dasatinib plus quercetin outperforming fisetin in both groups. When investigators treated young obese mice with senolytics, only dasatinib plus quercetin boosted urinary α-klotho levels.
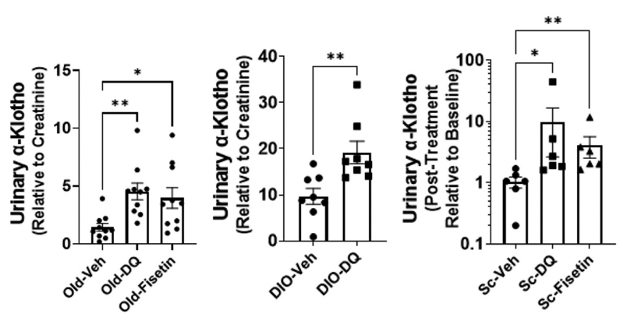
(Zhu et al., 2022 | eBioMedicine) Senolytics boost α-klotho in aged mice and young mice with obesity or receiving senescent cell transplants. The graphs show urinary α-klotho levels in (left) old mice, (center) young obese mice (DIO), and (right) young mice that received senescent cell transplants following treatment with placebo (veh), dasatinib plus quercetin (DQ), and fisetin.
Given that α-klotho synthesis heavily occurs in the brain, Zhu and colleagues also looked at α-klotho levels in the cerebellum and choroid plexus, two critical brain regions, in aged and young obese mice following senolytic treatment with dasatinib plus quercetin. As expected, dasatinib plus quercetin effectively raised brain α-klotho levels in both experimental groups, further indicating that senolytics, particularly dasatinib plus quercetin, restore declining α-klotho levels that occur with age or in senescence-linked diseases.
Dasatinib Plus Quercetin Raise α-Klotho in IPF Patients
Dasatininb plus quercitin is a well-established senolytic treatment, with studies even confirming its ability to enhance muscle and bone regeneration in mice. Although more clinical trials are needed to assess human translatability, Zhu and colleagues sought to determine whether dasatinib plus quercetin could increase α-klotho levels in patients with idiopathic pulmonary fibrosis (IPF) – a deadly age-related disease where senescence and declining α-klotho levels are key drivers of the disease. On top of successfully mitigating physical dysfunction in IPF patients with nine doses of dasatinib plus quercetin spanning three weeks, dasatinib plus quercetin also led to increases in urinary α-klotho. So, these findings further corroborate dasatinib plus quercetin as an effective senolytic treatment that can potentially be used to attenuate drivers of age-related diseases.
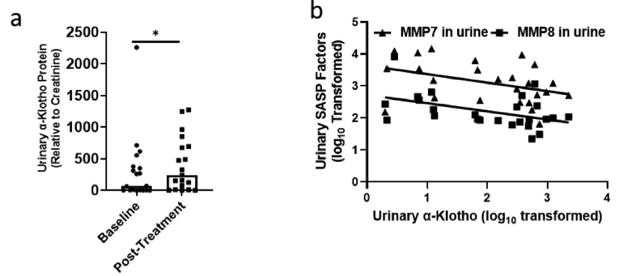
(Zhu et al., 2022 | eBioMedicine) Dasatinib plus quercetin raises urinary α-klotho in idiopathic pulmonary fibrosis patients. (A) Urinary α-Klotho levels in IPF patients increase post-treatment with dasatinib and quercetin. (B) Inverse correlation between levels of α-Klotho and SASP.
Updating the Anti-Aging Clinical Trial Toolkit
The Mayo Clinic researchers say that their results indicate that testing urinary α-Klotho could be a useful approach for monitoring effects of senolytics during clinical trials or, eventually, if senolytics are translated into clinical practice, in the course of treating patients. There are several advantages to a urine test compared to blood-based tests. Urine collections are less invasive than a blood draw and are probably less confounded by the possible effects of other proteins in the blood that might bind to α-Klotho.
Zhu and colleagues conclude, “Our study also opens a novel, translationally-feasible avenue for developing orally-active small molecules to increase α-Klotho, which may also be a useful biomarker for senescent cell burden or senolytic drug activity in clinical trials.”