Key Points:
- Senescent cells — dormant cells that promote tissue damage with aging — are elevated in the post-mortem brains of Alzheimer’s disease (AD) patients.
- An induced neuron (iN) model from AD patients recapitulates post-mortem tissue, including elevated senescent cells.
- Senolytics — drugs that selectively eliminate senescent cells — remove senescent cells from AD iNs.
Despite decades of research, no existing treatment stops the progression of AD. As early as the 90s, research suggested that senescent cells may contribute to AD, but this idea was dismissed because too many scientists believed neurons could not enter a senescent state. It is now known that senescent cells accumulate with age and contribute to many age-related disorders. Furthermore, today it is widely accepted that neurons can enter senescence.
Now, in the latest issue of Cell Stem Cell, scientists from the Salk Institute for Biological Studies demonstrate that senescent neurons are elevated in AD patients’ brains. Herdy and colleagues go on to show that iNs converted from AD patient skin cells model AD brain tissue and also exhibit elevated senescence. Finally, dasatinib and quercetin (D + Q) is shown to reduce senescent cells in AD iNs. Along with previous animal studies, these findings suggest that senolytics can help treat AD.
Senescent Neurons in Alzheimer’s Brains
Herdy and colleagues took post-mortem brains from 30 AD patients and 99 age-matched cognitively normal (NC) individuals and measured CDKN2A mRNA. The CDKN2A gene is considered to be the most specific marker of senescent cells. In AD brains, CDKN2A mRNA levels were increased in a region called the prefrontal cortex, which has an abundance of neurons.
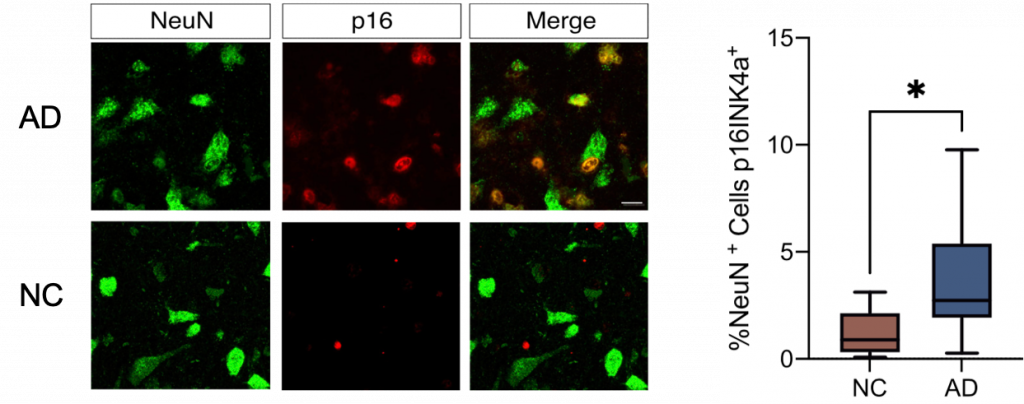
To determine if the CDKN2A gene was being activated in neurons specifically, prefrontal cortex tissue from another set of AD and NC individuals was examined. The CDKN2A gene codes for p16, a protein that can be measured with fluorescent staining. By showing that p16 fluorescence overlapped with fluorescent NeuN, a neuron-specific protein, the researchers demonstrated that senescent neurons were increased in AD brains.
Senolytics Remove Senescent Neurons
Since it is difficult to test drugs on post-mortem tissue, Herdy and colleagues tested the D + Q senolytic combo on induced neurons (iNs). To generate iNs, skin biopsies were collected from AD patients and non-demented individuals. Then, cells called fibroblasts from the skin samples were converted into neurons using a previously established process. A 3-fold increase in senescent neurons was found in AD iN cultures compared to iN cultures from non-demented individuals, suggesting that iNs can model neuronal senescence.
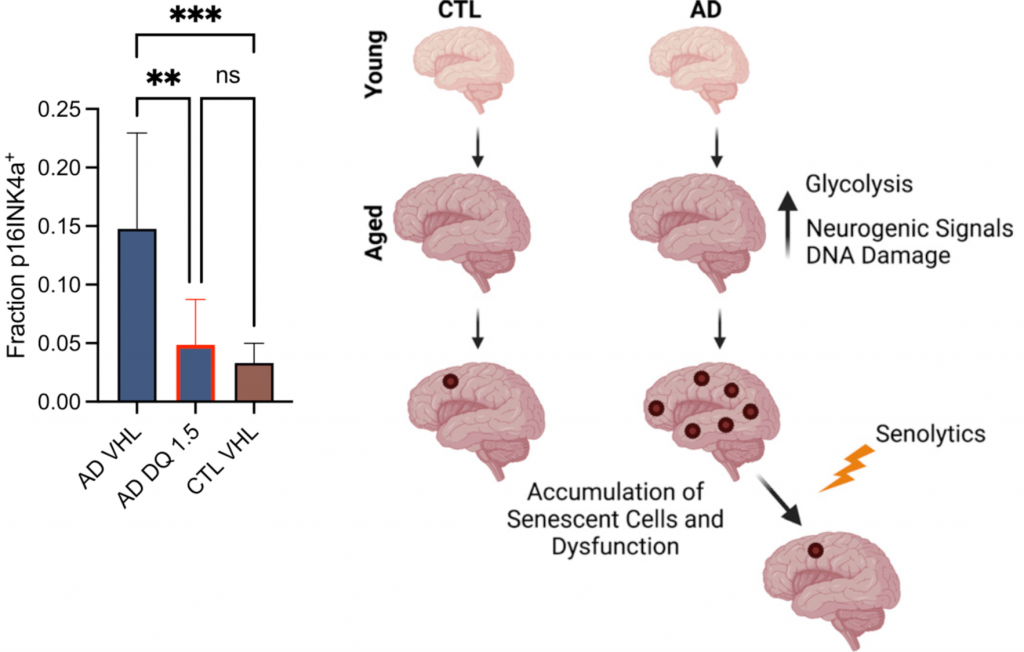
Clearing senescent cells from the brain of AD mouse models has previously been shown to alleviate cognitive impairment. The pharmacological elimination of senescent cells using D + Q has had similar results. Herdy and colleagues showed that D + Q could lower senescent neuron abundance in AD iN cultures to levels comparable to iN cultures from non-demented individuals. Together with previous findings in animal models, these results suggest that D + Q could alleviate cognitive impairment in AD patients.
Senescent cells can be beneficial for wound healing and preventing the spread of cancerous cells. However, if senescent cells are not cleared and allowed to accumulate, as with aging, they can cause damage to surrounding cells by secreting molecules collectively known as the senescence-associated secretory phenotype (SASP). The results of Herdy and colleagues provide evidence suggesting that these SASP factors are increased AD neurons, leading to increased brain inflammation.
“Our study clearly demonstrates that these non-replicating cells [neurons] are going through the deterioration process of senescence and that it is directly related to neuroinflammation and Alzheimer’s disease,” says senior author Dr. Rusty Gage.
Upcoming Clinical Trial for Senolytic Treatment of Alzheimer’s
Aging is the single highest risk factor for AD, so targeting the underlying source of aging — including senescent cells — may prove to be a viable treatment option. As such, in an upcoming clinical trial (NCTO4063124) called Senolytic Therapy to Modulate the Progression of Alzheimer’s Disease (SToMP-AD), Mayo Clinic scientists will give five early-stage AD patients 100 mg of dasatinib and 1000 mg of quercetin every two weeks for twelve weeks.
The outcome measures of the STomP-AD study will include senescence markers measured from the spinal fluid of the participants, cognitive function tests, and physical function tests like gait speed and grip strength. The brain will also be imaged via MRI to assess neurodegeneration. With the hope of D + Q leading to improvements in these outcome measures, a phase II clinical trial is already in the works. Therefore, it may not be too long until we use senolytics as a treatment option for AD.