Key Points:
- Treatment with D+Q reduces the proportion of age-associated cells from the testes of aged mice by nearly 10-fold.
- D+Q rejuvenates the growth of sperm stem cells, which can develop into mature, fully motile sperm cells.
Male fertility begins to decline after the age of 40 and is associated with decreased sperm counts. However, whether the accumulation of age-associated cells called senescent cells contributes to the hindrance of male fertility is unclear.
Now, researchers from Tokyo University in Japan report in iScience that senescent cells increase with age in the testes of mice. Furthermore, Ozawa and colleagues show that senolytics — compounds that remove senescent cells — bring the proliferation of sperm stem cells to more youthful levels, suggesting rejuvenation. With further studies, we may find that senolytics increase sperm cell counts in aged mice, which would enhance their reproductive potential.
Senolytics Promote Sperm Stem Cell Proliferation
In response to stressors like DNA damage and inflammation, our normally functioning cells can convert to senescent cells. Senescent cells no longer serve their original purpose and, at times, render surrounding cells dysfunctional. Furthermore, as senescent cells accumulate with age, they can drive age-related conditions, such as diabetes and cardiovascular disease.
A specific class of cells called endothelial cells (ECs), which encase blood vessels, seem to be particularly susceptible to senescence. Indeed, Ozawa and colleagues found an increased proportion of senescent ECs in the testes of aged mice compared to young mice. As testicular ECs play an essential role in controlling the function of sperm stem cells, senescent ECs may hinder fertility by impeding sperm stem cell function.
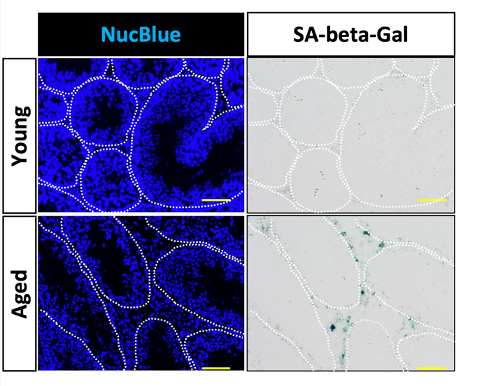
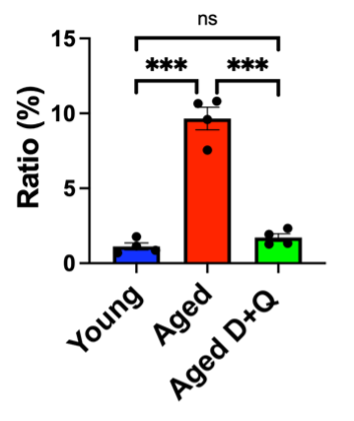
In addition to increased senescent ECs, Ozawa and colleagues also observed low sperm counts from the aged mice. Furthermore, the aged mice exhibited reduced fertility, as measured by diminished successful pregnancies with female mice. To determine if senolytics could potentially enhance fertility, the researchers took ECs from the testes of aged mice and treated the ECs with D+Q in a lab dish. As a result, the proportion of senescent ECs decreased by nearly 10-fold. This experiment shows that D+Q can remove senescent testicular ECs.
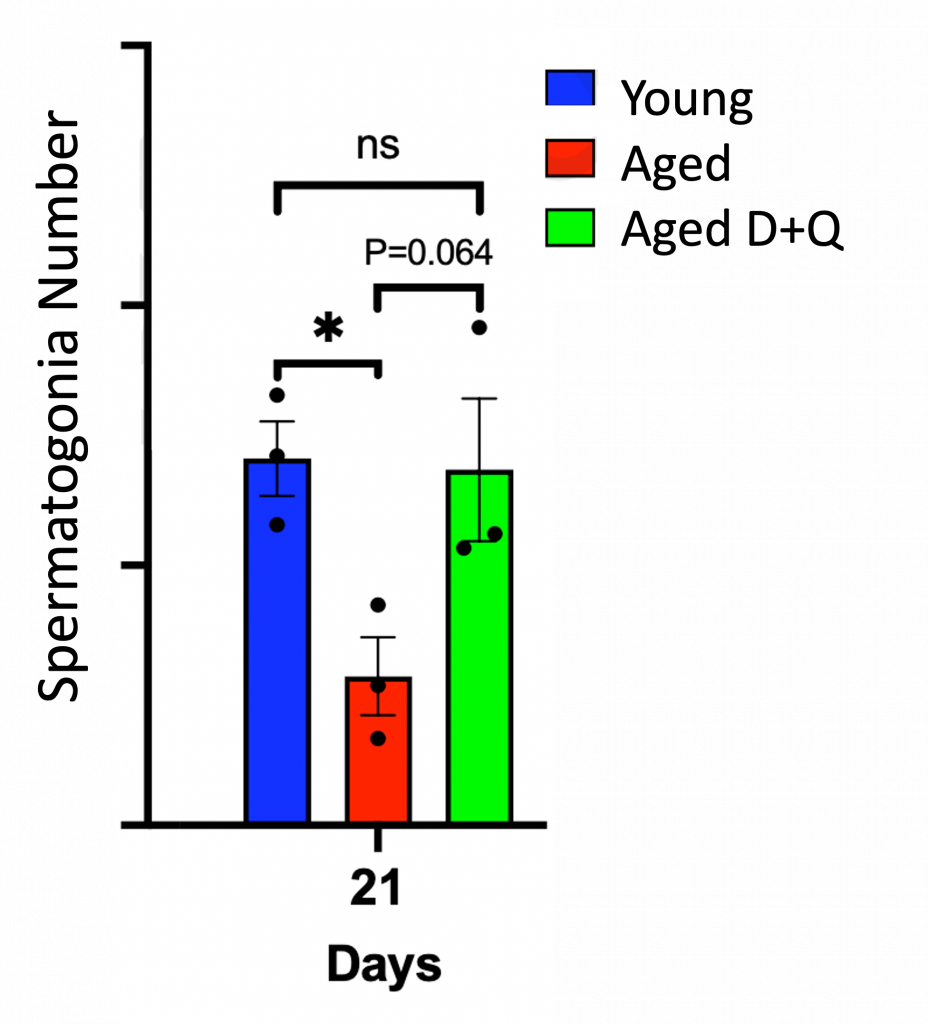
Sperm stem cells, referred to as spermatogonia, normally develop into full-fledged sperm cells with the support of ECs. However, since spermatogonia are surrounded by ECs in the testes, senescent ECs could potentially hinder the development of sperm cells by rendering spermatogonia dysfunctional.
To test this, Ozawa and colleagues took ECs from the testes of aged mice and treated them with D+Q. They then grew spermatogonia in the same dish with these D+Q-treated aged ECs. After 21 days, the growth of these spermatogonia reached numbers rivaling those grown with young ECs. These findings suggest that removing senescent ECs rejuvenates the proliferation of spermatogonia.
The Human Outlook
Overall, the findings of Ozawa and colleagues suggest that testicular ECs become senescent with age, which generates a less supportive environment for spermatogonia to proliferate. In turn, there are fewer sperm precursor cells available to generate mature sperm cells.

Whether this occurs in humans is unknown. Therefore, studies are needed to determine if senescent ECs occur in greater numbers within the testes of older vs younger males. Also, even in mice, it is still unclear whether senescent testicular ECs contribute to fertility hindrance. This could be shown by administering D+Q to aged mice and measuring their fertility rates. Overall, the question of whether senescent cells contribute to the hindrance of male fertility in humans remains unclear.