Lung fibrosis is the primary cause of death in industrialized societies and is one of the most feared pulmonary complications, with a clinically progressive yet unpredictable course. Early detection and timely prevention of this irreversible disease are the only available tools that resemble a cure; however, humans with progressive to advanced stages of this disease will undergo lung transplantation as a last resort.
Recent advances in lung fibrosis research have implicated a family of enzymes known as sirtuins (SIRTs) as potential therapeutic targets for regulating the progression of the disease. However, the amount of research elucidating the effects of sirtuins on lung fibrosis is scarce.
A thorough review of the regulatory role of sirtuins in lung fibrosis was published by a team of researchers from India. “We tried to delve deeper even into the regulatory of under-reported sirtuins on…pathways critical to lung fibrosis and indicate plausible implications therein,” said the authors. The authors suggested that there are numerous prospects for explanatory biological investigations to develop novel sirtuin-based treatment methods for lung fibrosis.
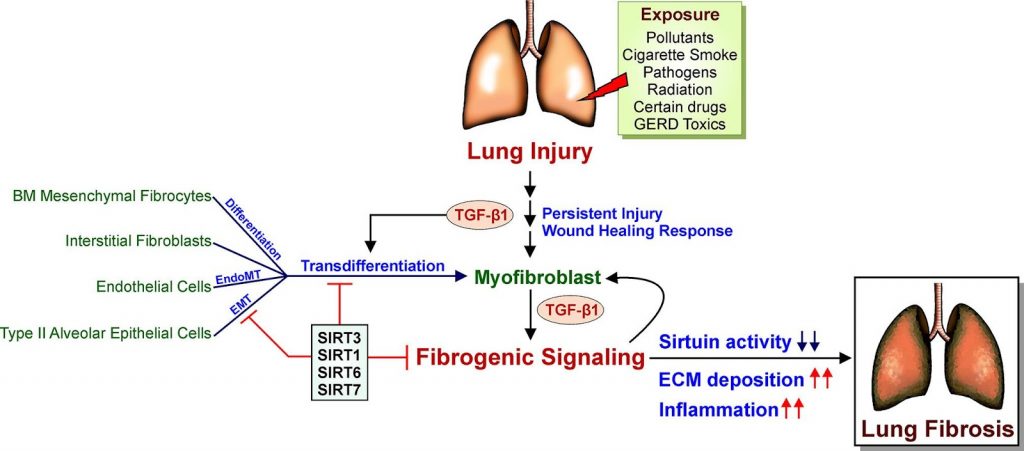
Sirtuins have been linked to age-related degenerative and inflammatory diseases, as well as nearly all kinds of organ fibrosis. Four of the seven sirtuins (SIRT1, SIRT3, SIRT6, and SIRT7) have been shown to protect against cardiovascular, pulmonary, and metabolic disorders such as fibrosis. However, the physiological roles of the remaining sirtuins (SIRT2, SIRT4, and SIRT5) in lung disorders remain unknown.
Overall, sirtuins appear to protect against lung fibrosis. Although no studies have successfully identified the specific functions of SIRT4 and SIRT5 in lung fibrosis, protective effects of these sirtuins may be assumed due to their favorable influence on the mitochondria, the cell’s powerhouses. A better understanding of these unknown features of sirtuin biology will likely lead to the discovery of new therapeutic targets for lung fibrosis. Moreover, scientists are calling for more research into the roles of SIRT4 and SIRT5 in lung fibrosis. SIRT2, the single sirtuin that fails to protect against lung fibrosis, may have a pro-fibrotic effect due to its proinflammatory effects seen in asthma. Based on the evidence for SIRT2’s negative connection with asthma, it’s possible that suppressing SIRT2 activity could be advantageous in the fibrotic environment as well.
The mechanism in which sirtuins are activated and regulated is a fascinating topic in regards to sirtuin-induced regulation of lung fibrosis. Due to the regulatory activity of these sirtuins, stimulation of SIRT6 and SIRT7 is likely to prevent lung fibrosis in addition to SIRT1 and SIRT3. Targeting SIRT1, SIRT3, SIRT6, and SIRT7 for therapeutic modulation have shown great potential because laboratory research in animals and cell lines, as well as data from humans with lung fibrosis, clearly suggest that sirtuins exhibit protective functions.
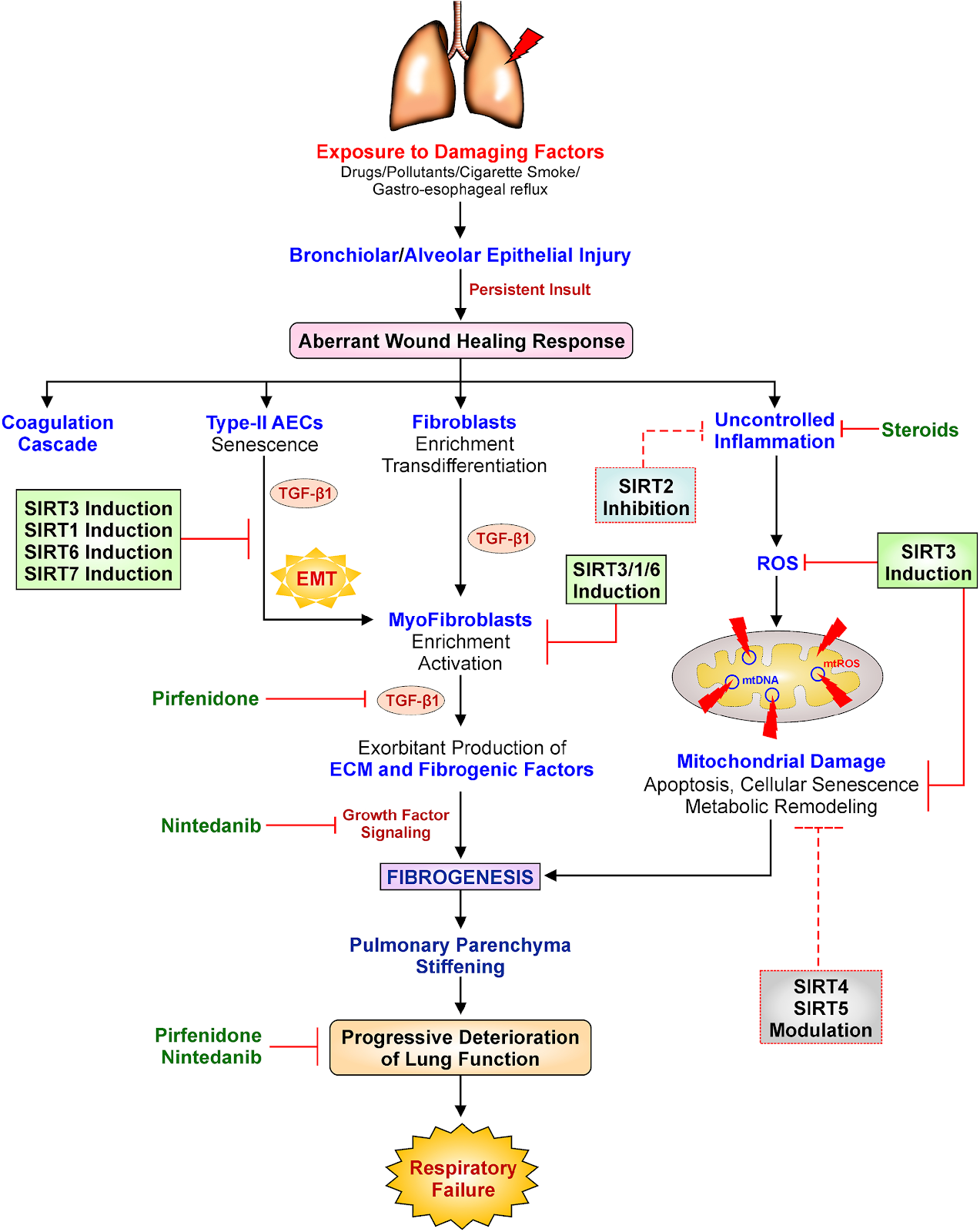
The investigators of this study also highlighted prospective therapeutic alternatives targeting sirtuins utilizing synthetic and plant-derived molecules that can aid researchers in developing new generation, low-cost, non-toxic sirtuin-based therapies to treat lung fibrosis. In this context, mice were reported to be protected against lung fibrosis after treatment with honokiol, a natural SIRT3 induces taken from Magnolia officinalis bark extract, and resveratrol, a natural SIRT1 inducer obtained from grapes. Due to the low bioavailability of resveratrol, different formulations with enhanced bioavailability, such as SRT501, resVida®, and Longevinex®, have been produced and commercialized as dietary supplements. These formulations are already commercially accessible for human consumption, but the US Food and Drug Administration has not approved them as medications due to herbs and dietary supplements not being closely regulated by the FDA.
While natural plant-derived substances have already gained attention as possible effective therapeutics, the development of small compound modulators of sirtuins is a promising field of lung fibrosis medical research. Therefore, the focus should always be on tactics that can enhance sirtuin inducers from native plant sources or bulk-scale manufacturing of synthetic activators, which can lead to the development of more affordable and effective medications.
While the majority of prospective therapeutic substances are evaluated in preclinical research utilizing experimental models, several have also been examined in clinical trials for various disorders. As a result, human clinical trials of these substances in lung fibrosis are required before humans can use them to treat diseases.
“In summary, the role of sirtuins in regulating pulmonary fibrosis is immense and unambiguous,” said the authors. “It depends on us as to what extent we can rationally exploit these marvelous therapeutic targets to overcome this deadly disease.”