Key Points:
- Old brain stem cells in aged animals lose their ability to replicate due to increased adhesions that restrict their movement.
- Using a drug that inhibits the formation of these adhesions boosts migration speed in cultured brain stem cells from aged brains.
- This same drug improves the generation of new neurons from these brain stem cells in old mice.
Scientists have discovered a way to reawaken dormant brain stem cells in older animals, which improves neuron production in their aging brains. Researchers from Stanford University discovered that as brain stem cells age, they lose their ability to migrate. This ability is crucial for the process of neurogenesis, which is the generation of new neurons. Even in an aged brain, neurogenesis can be stimulated by inhibiting this pathway and reducing adhesions, which provides brain stem cells with more mobility and thus allows neurogenesis to take place. This research, which was published in Nature Aging, identifies a therapeutic target that has the potential to reverse age-dependent defects and slow the aging of the brain.
Brain Stem Cell Niches
Niches are small, specialized pockets of replicative activity found throughout the adult brain. In these niches, regenerative brain stem cells generate new brain cells, primarily neurons (which are like the wires in our brains that send signals) and supporting cells called glial cells. As we get older, the stem cell niches in our brains lose some of their ability to support regeneration, which reduces our capacity to repair damaged neurons and our ability to retain normal levels of sensory and cognitive function.
Within the hippocampus, in a region known as the subventricular zone (SVZ), there is a brain stem cell niche that plays an important role in learning and memory. These dormant brain stem cells have the potential to become active and generate progenitors that then migrate forward in the direction of the olfactory bulb, which is the primary sensory center in mice that is responsible for the processing of smells, to produce new neurons. The offspring of these activated brain stem cells migrate to the sites of injury to reduce the damage’s severity by producing new neurons and specialized glial cells. Both the regenerative potential and repair abilities of the SVZ neurogenic region decline with age.
Old Activated Brain Stem Cells Exhibit Defective Migration
Previous studies have shown that aging affects gene activity in the SVZ neurogenic niche and that changes to the epigenome, which are chemical modifications to DNA that regulate gene activity, play an important role in both stem cells and the aging process. However, the age-dependent epigenomic changes that take place in the various cell types that make up the neurogenic niche in animals and humans are still a mystery.
The co-lead authors of this study, Robin W. Yeo and Olivia Y. Zhou, collaborated with other researchers from the lab of Anne Brunet at Stanford University to profile and compare the epigenomes of young and aged brain stem cells taken from the SVZ. They discovered that aging has opposing effects on the global epigenome landscape of quiescent and activated brain stem cells (and brain stem cell progeny) in mice. In particular, there were significant differences in the epigenome in the regulatory region for gene activity that is essential for cell adhesion and migration.
These findings were visually confirmed with live imaging studies in cultured brain stem cells and mice, showing that old activated brain stem cells exhibit migration defects as they age. This, in turn, affected the location of the aged brain stem cells and their offspring in the brains of mice. Based on these findings, it appears that aging may reduce the capacity of activated brain stem cells and the offspring of these cells to exit the brain stem cell niche and travel to their final destination.
Rock Inhibition Boosts Old Activated Neural Stem Cell Migration and Proliferation
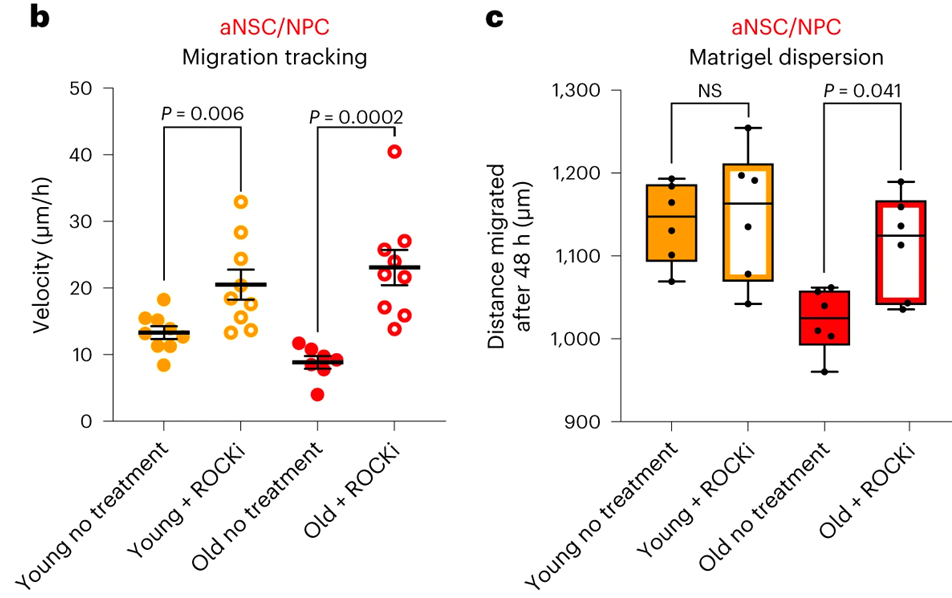
Next, they wanted to find a molecular target that could counteract the increased adhesion strength observed in activated brain stem cells. This would be done to restore the age-related mobilization of old activated brain stem cells and their offspring out of the niche, which would ultimately lead to an improvement in neurogenesis in the older brain. An in-depth investigation into the underlying mechanisms of this phenomenon revealed that aging not only increases the number of adhesions but also the adhesion strength of activated old brain stem cells. They then determined that the most important component of this process was a pathway known as the ROCK pathway.
Old activated brain stem cells that had been cultured were treated with a small molecule ROCK inhibitor (Y-27632) to see if they could reverse the increase in cell adhesion normally associated with aging in old activated brain stem cells. This was done to determine whether or not the ROCK pathway could be utilized to reverse the effect. They discovered that inhibiting ROCK could get rid of adhesions in activated brain stem cells taken from older mice. In addition, the inhibition of ROCK resulted in a speed increase in the migration of these cells.
Finally, the Stanford researchers investigated whether or not the inhibition of ROCK in cell culture could be applied to animals. With the help of a mini-osmotic pump, the ROCK inhibitor was injected into the lateral ventricles of the elderly mice. These ventricles—which carry a clear, colorless, watery fluid that flows in and around your brain and spinal cord called cerebrospinal fluid—are located near the subventricular zone neurogenic niche.
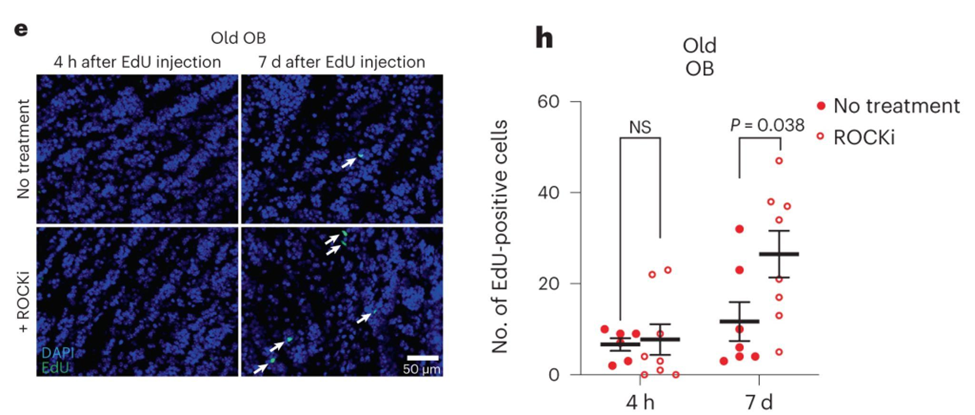
It is interesting to note that the delivery of ROCK inhibitors into the ventricles resulted in a significant increase in the migration of activated brain stem cells away from the ventricle in a manner that was analogous to the activity of young brain stem cells. In addition, the inhibition of ROCK increased the number of new neurons found in the olfactory bulbs of older mice. Therefore, inhibiting the ROCK pathway might be a good strategy for improving the migratory properties of old activated brain stem cells (and their offspring) and for boosting neurogenesis in older brains.
These findings have significant repercussions for understanding the role that adhesion and migration play in the process of brain stem cell aging, and they point to ROCK as a potential therapeutic target for restoring age-dependent defects that manifest in older people. ROCK inhibitors are safe to use in humans, and research on them has already begun in the context of neurodegenerative diseases and stroke. All in all, it seems that these inhibitors have the potential to be used to treat other age-related conditions that affect the brains of older people.