Key Points:
- The basic units of female reproduction decline with age in women, leading to infertility.
- Stem cell therapy counteracts this decline and reduces molecular drivers of aging such as inflammation in monkeys.
- One of the monkeys treated with stem cells successfully became pregnant.
Menopause marks the end of a woman’s reproductive lifespan, usually around age 50. However, delaying the onset of menopause may promote a longer life. For every 1-year delay in menopause onset, there is a 2% decrease in mortality rates, according to a study. This means that delaying menopause by 5 years could lower the risk of early death by 10%. Moreover, menopause has been shown to accelerate biological aging, suggesting that delaying menopause can maintain a typical aging trajectory.
With advances in the field of aging biology, scientists are now finding that delaying menopause may potentially be achieved with stem cells. Stem cells have the remarkable capacity to regenerate organs and tissues, making them perfect for counteracting degenerative aging — the gradual deterioration of our organs and tissues. As published in Cell Discovery, researchers from the Chinese Academy of Sciences in Beijing have found that stem cell therapy extends the reproductive lifespan of cynomolgus monkeys. These findings have far-reaching implications for the longevity of women.
Ovarian Aging in Humans
The ovaries house the basic units of female reproduction called primordial follicles, each containing an unfertilized oocyte. Women are born with a finite number of primordial follicles and, with each menstrual cycle, they diminish over time. This age-related decrease in follicles is called ovarian aging and ends in menopause, whereby menstrual cycles cease and there is no longer a reserve of viable follicles.
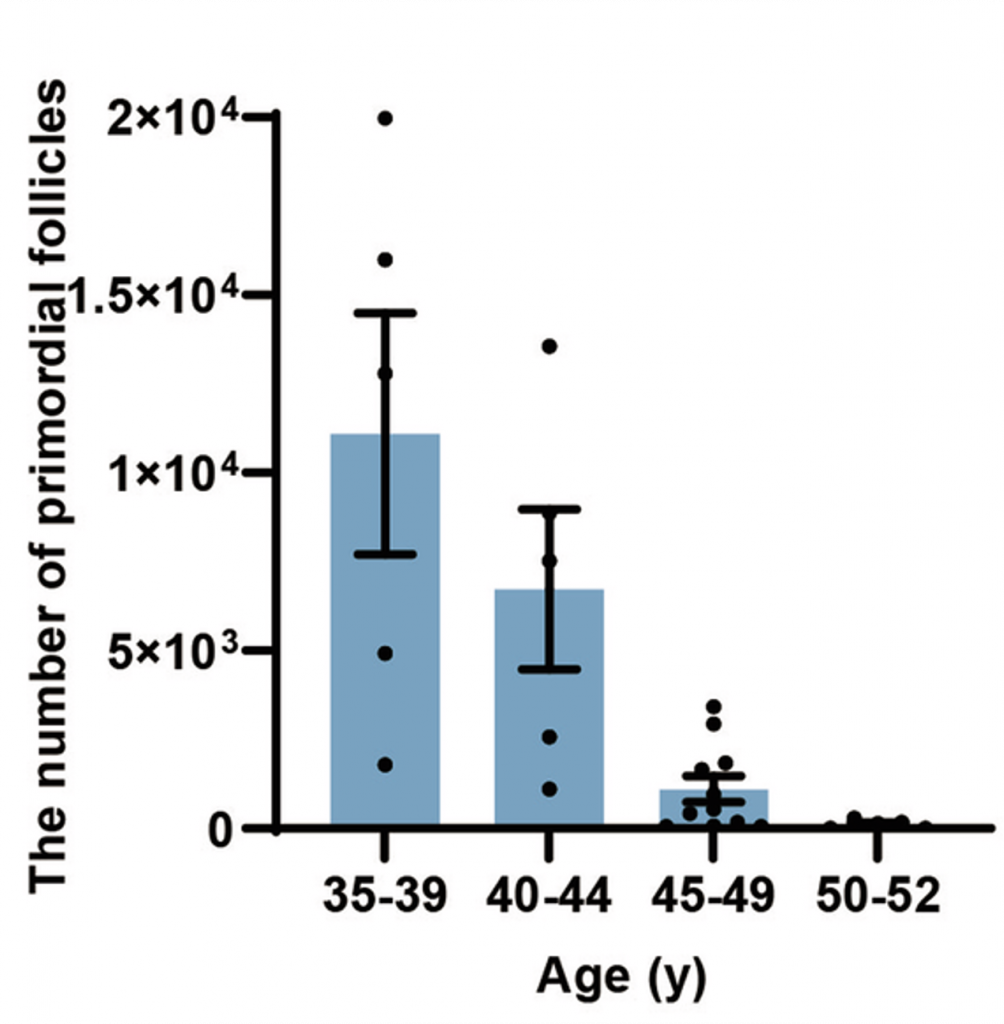
To confirm this, the researchers measured primordial follicle numbers from women aged 35 to 52. The women were separated based on age in five-year intervals (i.e., 35–39 years, 40–44 years, 45–49 years, and 50–52 years). The results showed an age-related reduction in primordial follicles with the dramatic drop occurring after the age of 44, whereby primordial follicle numbers were reduced to 1,019 from 6,728. These findings demonstrate ovarian aging in an Asian population for the first time.
Stem Cell Therapy Prolongs Reproductive Lifespan
Previous studies have shown that stem cell therapy promotes fertility in animal models and women with premature ovarian insufficiency, a condition that mimics menopause and occurs before the age of 40. One study even showed that transplanting stem cells from young monkeys into old monkeys reverses ovarian aging. However, the applicability of M-cells — stem cells that can be manufactured on a large scale — on reproductive aging has not been tested until now.
The researchers selected ten cynomolgus monkeys that were approaching menopause to test the M-cells. Seven of the monkeys were injected with M-cells, while the other three were injected with only saline. The monkeys were injected a total of two times with a month in between injections. The monkeys were then assessed over an 8-month follow-up period. Over this follow-up period, the monkeys remained healthy, suggesting the stem cell treatment is safe.
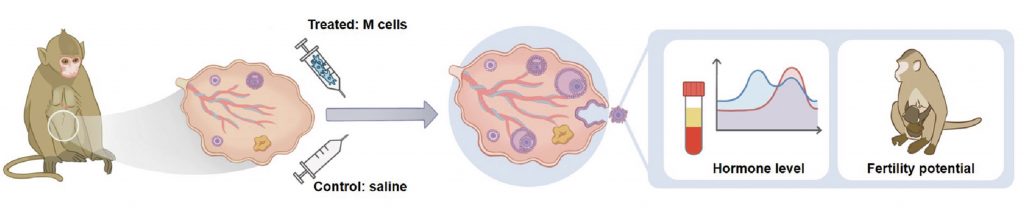
The researchers found that the hormone (i.e., estrogen and progesterone) levels of monkeys injected with M-cells were significantly higher than monkeys injected with saline. Additionally, to assess fertility potential, the monkeys were injected with experimental hormones that stimulate follicle growth. As a result, the researchers found that M-cell therapy increased the number of oocytes within the ovaries. These findings demonstrate that M-cell therapy improves hormone levels and enhances fertility potential in monkeys.
Importantly, the researchers tested whether the monkeys could conceive naturally. To test this, they placed the monkeys into a cage with male monkeys for two months. As a result, one of the M-cell-treated monkeys became pregnant and eventually delivered a healthy baby, which is now over 3-years-old. These findings demonstrate that stem cell therapy is capable of prolonging reproductive lifespan, at least for some monkeys.
Targeting the Molecular Drivers of Aging
The researchers at the Chinese Academy of Sciences showed that M-cell therapy reduced inflammation and oxidative stress in the ovaries of the treated monkeys. Inflammation and oxidative stress are molecular drivers of degenerative aging, and these processes underlie nearly every chronic age-related disease, including cardiovascular disease and neurodegenerative disease.
Considering that stem cell therapy will likely not be approved for the treatment of aging, including ovarian aging, in the near future, there may be alternatives. NAD+ precursors, including nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) have been shown to reduce inflammation and oxidative stress in multiple organs and tissues, including the ovaries. Therefore, while human studies are necessary, NAD+ precursors may potentially combat ovarian aging.