Key Points:
- A compound derived from broccoli called sulforaphane induces weight loss and improves blood sugar levels in obese mice.
- Sulforaphane prevents weight gain in lean mice fed a high-fat diet.
Two billion people in the world are either obese or overweight. That’s over a quarter of the world’s population. As the rate of obesity – and its associated coexisting diseases, including diabetes and high blood pressure – continue to rise, scientists are searching for medications that may be able to combat it with minimal side effects. A study out of Vanderbilt University shows that a compound found in cruciferous vegetables – known as sulforaphane – leads to weight loss, decreased food intake, and improved glucose parameters in diet-induced obesity mouse models. “Our findings argue for clinical evaluation of sulforaphane for weight loss and obesity-associated metabolic disorders,” the researchers wrote.
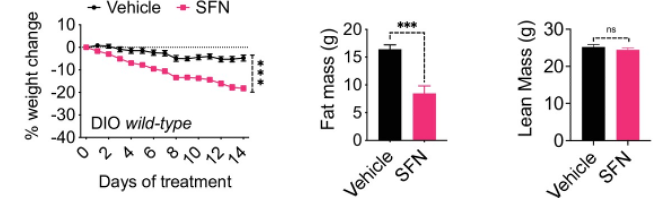
(Cakir et al. 2022 | eLife) Sulforaphane induces weight and fat loss in obese mice. Obese mice fed treated with sulforaphane had more weight loss (left) and less fat mass (mice) than untreated obese mice. Sulforaphane treatment did not affect lean mass (right).
Sulforaphane Treats Obesity and Prevents Weight Gain
Cakir and colleagues took obese mice and injected with sulforaphane (5 mg/kg/day) for 2 weeks. These sulforaphane-treated obese mice lost 14% more weight than the untreated obese mice. These mice also demonstrated reduced food intake, decreased fat stores, and better fasting glucose levels. However, these results were not seen in lean mice given sulforaphane treatment, which the researchers suggest is because sulforaphane requires excess fat to work. Sulforaphane treatment seems to work by decreasing the production of fatty acids in the liver and fat tissue and increasing the utilization of fatty acids as an energy source, allowing for only fat mass to be affected and not lean mass.
Additionally, when lean mice were fed a high-fat diet and treated with sulforaphane, the mice did not become obese. This effect was dose-dependent, where higher sulforaphane doses led to a greater decrease in caloric intake and less weight gain. Furthermore, treating lean mice with leptin plus sulforaphane – even without adding the high-fat diet – led to weight loss and decreased food intake, indicating that sulforaphane increased the effects of leptin and the body’s sensitivity to it.
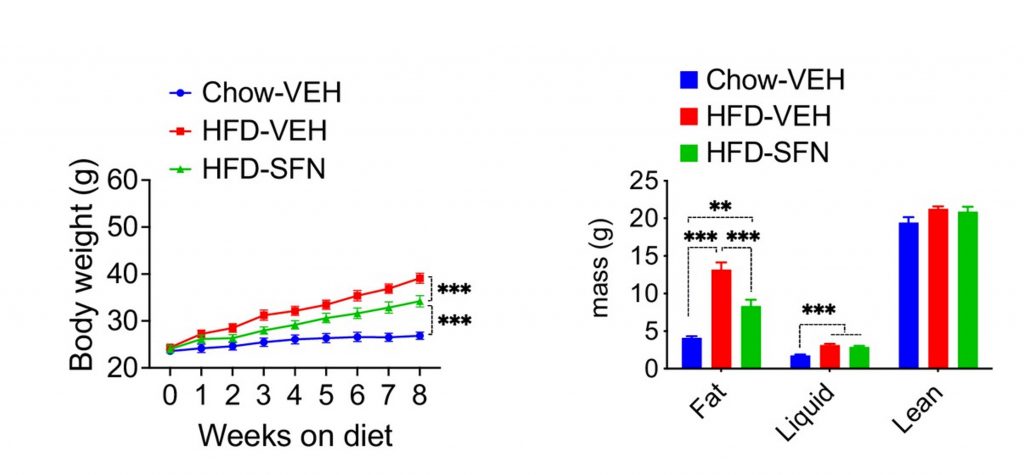
(Cakir et al. 2022 | eLife) Sulforaphane prevents weight and fat gain in lean mice fed high-fat diets. Lean mice fed high-fat diets (HFD) also treated with sulforaphane did not gain as much weight (left) or fat mass (right) as those fed
high-fat diet alone.
How hungry we feel and the amount of food we eat is regulated by hormones like leptin, which inhibits the feeling of hunger. In fact, diet-induced obesity often manifests as a leptin-resistant state, in which there is too much leptin in the bloodstream and the body becomes resistant to the effects of leptin (a state known as hyperleptinemia), which would normally help the body maintain a healthy weight. Increasing the body’s sensitivity to leptin is a possible consideration in the fight against obesity.
Since obese mice have hyperleptinemia, the investigators next looked at mice genetically modified to have no leptin. After sulforaphane treatment, the leptin-deficient mice did not have weight loss, decreased caloric intake, or improved glucose measures. However, restoring leptin, allowed the mice to lose weight and decrease their food intake, demonstrating that sulforaphane acts on leptin for its anti-obesity effects.
Sulforaphane – the Next Anti-Obesity Medication?
This study demonstrates how sulforaphane increases weight loss in obese mice and decreases their food intake, along with improving glucose parameters, indicating that sulforaphane may be the answer to the obesity epidemic. The ability of sulforaphane treatment to prevent weight gain in lean mice fed a high-fat diet may also be beneficial to Americans, given their generally high-fat diets. However, studies of sulforaphane in humans have indicated that it has a 10-fold lower efficacy in humans than in mice, and a safety study of sulforaphane in obese, diabetic patients showed that while it was well-tolerated and significantly reduced blood glucose, it had no effect on body weight. Thus, more research is needed to fully ascertain whether sulforaphane – or its more stable precursor, glucoraphanin – are the answer to the obesity epidemic.