Key Points:
- Treatment with THBru improves heart function, reduces fibrosis (scarring), and decreases senescent (aged) cells in a mouse model for aging.
- THBru delays cardiac aging by activating mitophagy – the clearance of defective/dead mitochondria.
Removing damaged cellular components, such as defective mitochondria via mitophagy, is vital to thwarting energy depletion, oxidative stress, and inflammation, all of which increase susceptibility to age-related diseases like cardiovascular disease. However, aging can compromise mitophagy, ultimately increasing the risk of these diseases. Thus, scientists have tasked themselves with identifying potent mitophagy activators that can potentially extend longevity and delay aging.
In a new study published in Acta Pharmocologica Sinica, Wang and colleagues explore the effects of THBru on cardiac aging in mice modeling accelerated aging. The investigators found that aged mice treated with THBru exhibit superior heart function, less cardiac fibrosis, and fewer senescent cells in heart tissue. What’s more, the findings suggest that THBru’s cardioprotective effects are mediated by mitophagy activation.
THBru Improves Diastolic Function, Reduces Fibrosis, and Eliminates Senescent Cells
To induce accelerated aging, the investigators treated mice with a sugar called D-galactose (D-gal). Following D-gal treatment, the research team treated mice with 50 mg/kg/day of berberine (BBR) or its derivative THBRu (low dose: 25 mg/kg/day or high dose: 50 mg/kg/day) and looked at heart parameters indicative of healthy aging: diastolic function – which helps regulate blood filling the heart’s lower chambers(ventricles) – and fibrosis. The findings showed that diastolic function was greatest in mice treated with a high dose of THBru. Furthermore, high-dose THBru treatment led to the most significant reduction in fibrosis, a hallmark of cardiac aging that increases the risk of heart failure.
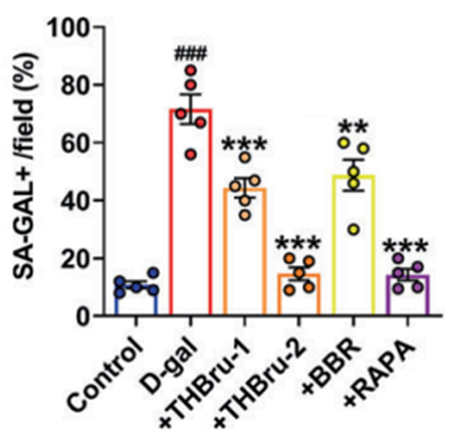
Senescent cells are growth-arrested cells that accumulate in all major organs of the body upon aging and are a major determinant of organ failure. Accordingly, Wang and colleagues tested whether THBru could eliminate senescent cells in the heart tissue of aged mice. The results showed that heart tissue treated with a high dose of THBru contains significantly fewer senescent cells than heart tissue treated with BBR or low-dose THBru, demonstrating that high-dose THBru treatment is most effective at limiting senescent cell burden. Taken together, the findings suggest that high-dose THBru attenuates features of cardiac aging.
THBru Delays Cardiac Aging by Promoting Mitophagy
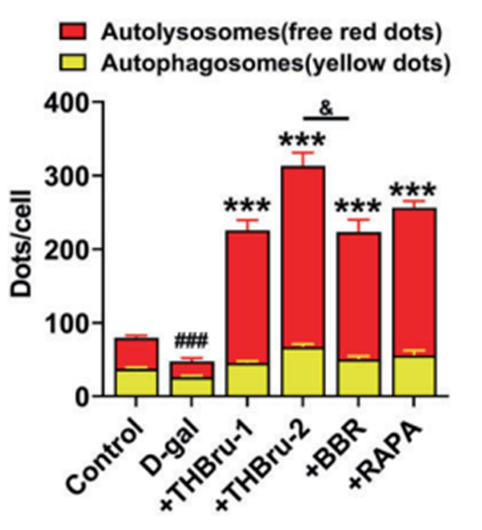
Wang and colleagues proceeded to investigate the underlying mechanisms behind THBru’s cardioprotective effects. As mentioned earlier, mitophagy is an essential biological process that helps maintain heart function, and the investigators showed that untreated aged mice with increased fibrosis and senescent cells also have impaired mitophagy. With this in mind, Wang and colleagues wanted to see if THBru’s cardioprotective effects resulted from activating mitophagy.
The findings showed that treatment with low-dose THBru activates mitophagy, reduces oxidative stress, and decreases senescent cells in the heart tissue of aged mice. However, high-dose THBru, which promoted greater mitophagy than low-dose THBru, led to even less oxidative stress and fewer senescent cells, demonstrating that THBru’s cardioprotective effects are mediated by increased mitophagy activation.
Mitophagy For Longevity
Researchers continue to unravel the longevity benefits of maintaining a healthy pool of mitochondria via mitophagy upon aging. What makes this field of research even more exciting is the abundance of mitophagy-inducing compounds, which sometimes hold multiple anti-aging properties. One such molecule is nicotinamide mononucleotide (NMN), which has been shown to mitigate stroke in rats and reduce neuron cell death in mice by activating mitophagy. Notably, NMN is a potent precursor to the life-preserving enzyme nicotinamide adenine dinucleotide (NAD+), which plays a vital role in delaying age-related diseases by promoting energy production and DNA repair. Another mitophagy activator with diverse anti-aging properties is urolithin A, which has been shown to improve muscle strength in older adults and boost physical performance in overweight adults. All things considered, mitophagy appears to have a definitive role in helping maintain overall health and delaying critical aging features.