Key Points:
- NR treatment reduces the proportion of stem cells that become mature fat cells.
- Treatment with NR increases genes associated with maintaining stem cell function.
- NR reduces the number of stem cells with excessive levels of reactive oxygen species (ROS) — molecules associated with accelerating aging.
While many of us would like to shed the pounds, our body fat is essential for survival. Amongst its many benefits, fat (adipose) tissue helps prevent dietary fat from getting into our organs. However, age-related fat loss negates this diversion, leading to the deposition of fat in our liver and muscles, which may contribute to age-related liver and muscle disease.
Now, researchers from the University of Pittsburgh may have found a way to combat age-related fat loss. As reported in Pharmaceuticals, Chinnapaka and colleagues show that NR improves the function of fat-derived stem cells, which is to ultimately regenerate fat tissue. They also show that NR, an NAD+ booster, reduces ROS levels within fat-derived stem cells, which may be how NR exerts its effects.
“The emerging field of NAD therapeutics offers a promising avenue for combating age-related decline in stem cell functionality. By targeting NAD homeostasis, researchers aim to develop interventions that can restore the youthful characteristics of stem cells and enhance their regenerative abilities,” said the authors.
NR Keeps Stem Cells as Stem Cells
Within our fat tissue are stem cells capable of transforming into mature fat cells, bone cells, or cartilage cells. Chinnapaka and colleagues isolated some of these stem cells from fat tissue donated by females undergoing plastic surgery. They then assessed the proclivity of the stem cells to become mature fat cells in response to NR.
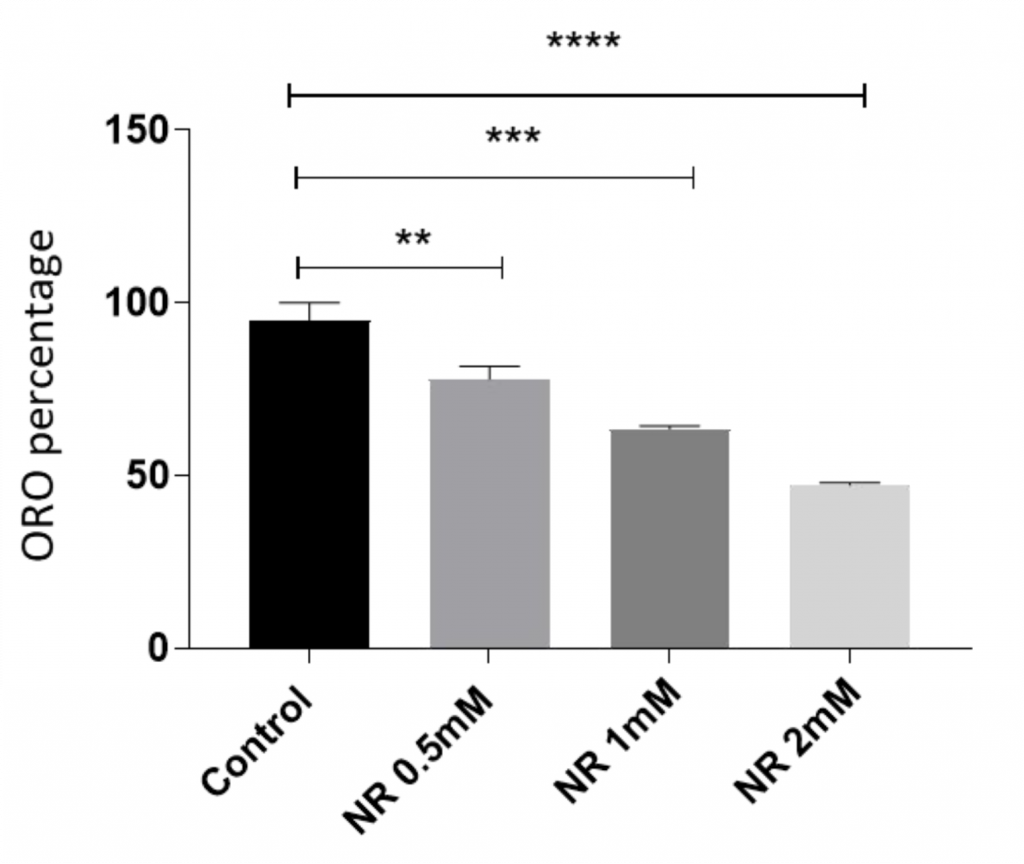
To do so, the researchers first chemically induced stem cell transformation then stained the cells with a dye called Oil-Red-O, which stains only mature fat cells the color red. The results showed that NR reduced the proportion of red cells, suggesting NR blocks stem cell transformation into mature fat cells.
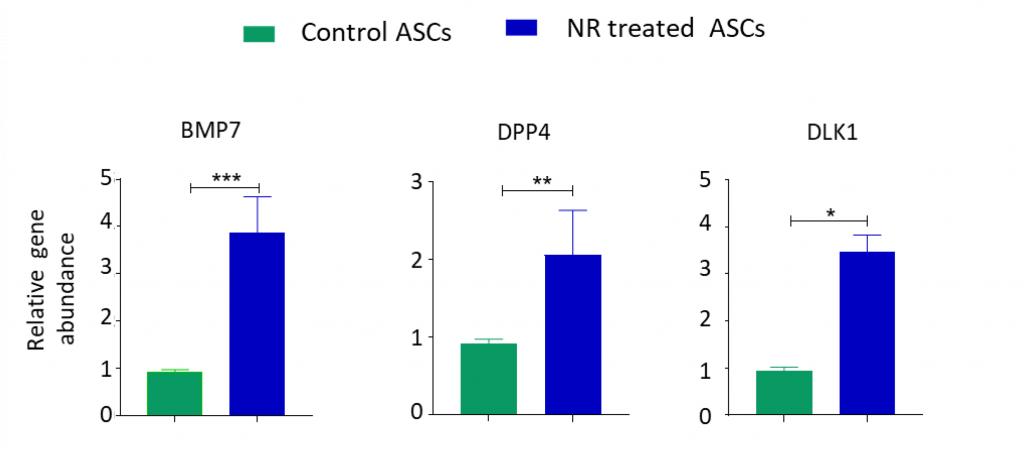
That NR reduces the proportion of stem cells capable of becoming fat cells suggests that NR promotes stemness — the ability of stem cells to duplicate and transform into different cell types, which is their function. To test this, Chinnapaka and colleagues measured genes associated with stemness in response to NR. They found that NR treatment resulted in a 2- to 3-fold increase in stemness genes, suggesting that NR promotes stemness.
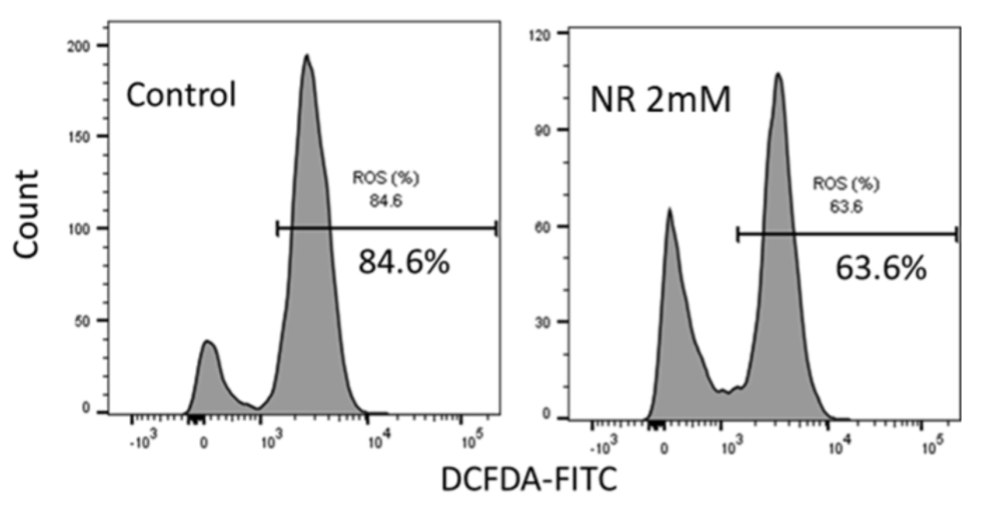
Loss of stemness is thought to be due to oxidative stress — a hallmark of aging caused by excessive levels of ROS. To determine if NR promotes stemness by reducing oxidative stress, Chinnapaka and colleagues counted the number of stem cells with high ROS levels in response to NR. The results showed that NR reduced the number of stem cells with high ROS levels by about 33%, confirming that NR reduces oxidative stress.
Overall, the findings of Chinnapaka and colleagues suggest that NR maintains stemness by reducing oxidative stress in fat-derived stem cells. Notably, oxidative stress drives stem cells to transform into mature fat cells, leading to senescence and cell death. The death of fat cells causes fat tissue loss, which cannot be regenerated without properly functioning stem cells.
“The failure of adipose (fat) tissue regeneration with age is a key contributor to the development of age-associated metabolic disorders. Further studies and clinical trials are necessary to explore the full potential of NAD-based therapies in combating age-related adipose tissue degeneration and promoting healthier aging,” the authors conclude.
Could Combining Senolytics with NAD+ Precursors Fully Rejuvenate Our Stem Cells?
With aging our stem cells tend to succumb to senescence — a dormant state whereby the cell no longer serves its function, but instead promotes the dysfunction of surrounding cells. Therefore, senolytics — compounds that selectively eliminate senescent cells — could get rid of senescent stem cells and counter age-related conditions. For example, the senolytic ABT-263 has been shown to counter arthritis by restoring stem cell function.
Moreover, the findings of Chinnapaka and colleagues suggest that an NAD+ precursor can maintain stem cell stemness. It follows that combining a senolytic with an NAD+ precursor could simultaneously remove dysfunctional stem cells while maintaining the health of functioning stem cells. However, this has not yet been tested.