Muscle mass and strength in humans begin to decrease around age 40, and muscle mass is reduced by up to 50% when humans reach their 80s. Evidence suggests that these reductions in muscle mass and strength promote the development of sarcopenia, a syndrome that induces progressive decline of muscle and strength. Furthermore, this syndrome is the leading cause of diminished function and privation of independence in older adults. Since the global population continues to age, scientists are motivated to develop safe and effective therapeutics to attenuate age-related sarcopenia.
In a recent study published in Scientific Reports, Sen and colleagues from Indiana University School of Medicine investigated the effects of urolithin A, a substance utilized in ancient Indian medicine, on aged-muscle, with the goal of curing this disease. Investigators noted that oral supplementation of urolithin A in mice led to increased cellular concentrations of an essential compound called nicotinamide adenine dinucleotide (NAD+), enhanced energy production in cells, and activation of angiogenesis, the formation of blood vessels, in muscle tissue. “This work lays the foundation to future work testing the effect of [urolithin A] supplementation on sarcopenia and its outcomes,” stated Sen and colleagues in their publication.
Urolithin A serves as a nutritional supplement, and Shilajit, a sticky substance located in rocks of the Himalayas, is known to contain copious amounts of urolithin A. Gradual plant decomposition over centuries formulates Shilajit, and this supplement is still utilized in traditional Ayurvedic medicine, which was developed over 3,000 years ago in India and is still considered one of the world’s most ancient systems of medical healing. Furthermore, studies have suggested that supplementing urolithin A during aging may provide a myriad of health benefits, such as elevating testosterone levels in men and enhancing muscle function. “In this work, we sought to understand the mechanism of action of orally supplemented [urolithin A] on limb skeletal muscles,” said Sen and colleagues in their publication.
Results showed that onset muscular aging in mice could be hindered through supplementation of urolithin A. Investigators began oral treatment with a urolithin A solution (10 mg/kg) in mice aged 12 weeks, and treatment continued until mice were aged 16 weeks. The obtained data suggested that 28-week-old mice resembled humans aged between 35 and 40 years old. In comparison to 28-week-old mice that were not supplemented with urolithin A, the muscles in mice that underwent urolithin A supplementation exhibited significantly lower levels of molecular markers of aging, which indicated that aging slowed down.
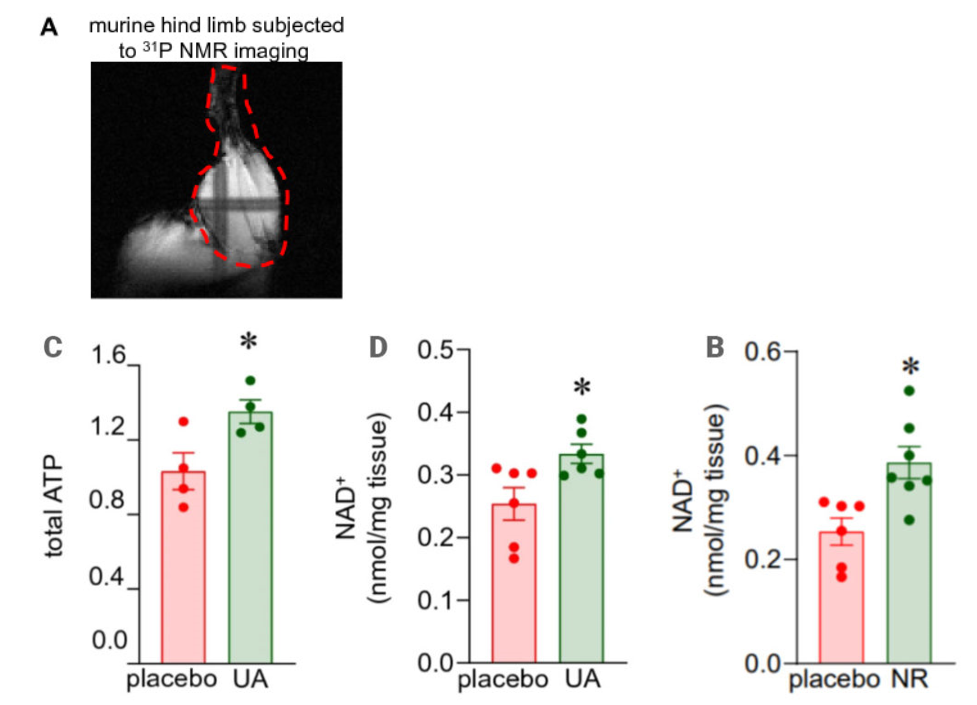
Due to the observed decrease in molecular markers of aging in muscle after urolithin A supplementation, investigators decided to evaluate if the generation of energy in old muscle tissue was affected by urolithin A supplementation. Sen and colleagues showed that supplementing mice with urolithin A induced higher levels of adenine triphosphate, the primary source of cellular energy, and elevated levels of NAD+, an essential compound for producing cellular energy. In addition, the data suggested that the observed elevated levels of NAD+ after urolithin A supplementation were comparable to the effects of mice receiving five-fold larger dosages of nicotinamide riboside (NR), a common precursor of NAD+.
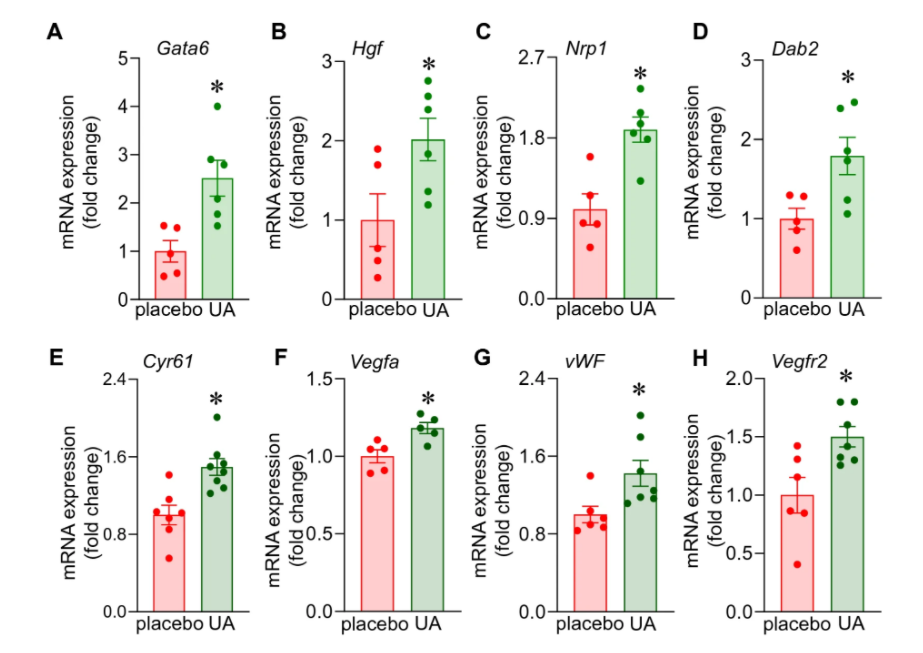
To investigate urolithin A’s molecular mechanism of action in muscle tissue, Sen and colleagues conducted a genetic analysis to elucidate the metabolic pathways that become triggered in muscle tissue upon urolithin A supplementation. Genetic activity was found to be increased in molecular pathways that produce blood vessels.
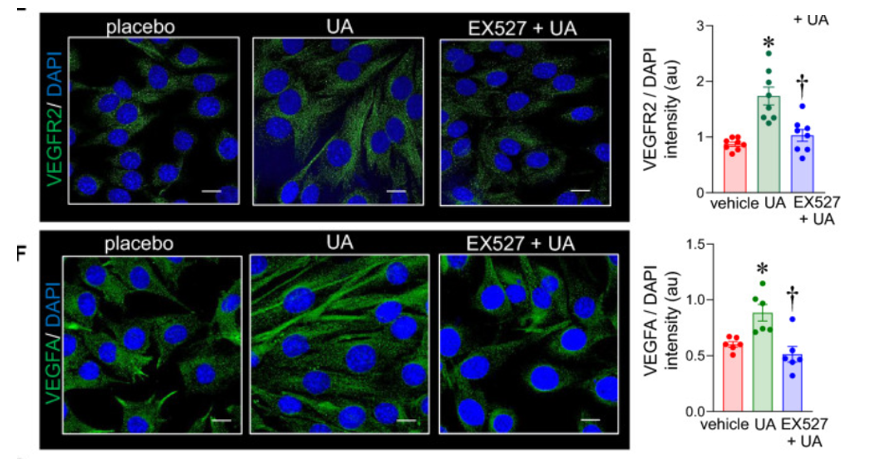
The research team then obtained evidence that sirtuin 1 was the compound responsible for inducing elevated blood vessel production in muscle tissue after urolithin A supplementation. Sirtuin 1 is known to facilitate anti-aging effects, and its ability to break off molecular tags called acetyl groups allows for the activation of various proteins. Further analysis found that urolithin A supplementation induced higher levels of sirtuin 1. Therefore, Sen and colleagues proceed with incorporating selisistat EX527, a sirtuin 1 inhibitor, with urolithin A in their muscle cell treatments. Results showed that this combination treatment led to decreased levels of molecular markers of angiogenesis. Ultimately, the obtained data suggest that urolithin A induces angiogenesis in muscles via a sirtuin-dependent mechanism.
To achieve optimal muscle and physical function, angiogenesis must occur. The team of scientists found that supplementing aged mice with urolithin A reduced markers of aging, induced angiogenesis in muscle tissue, and enhanced energy production in cells. If these effects could be reproduced in humans upon urolithin A supplementation, this supplement could have the potential to serve as a therapeutic to attenuate age-related sarcopenia.
“This work provides maiden evidence demonstrating that urolithin A supplementation bolsters skeletal muscle ATP and NAD+ levels causing upregulated angiogenic pathways,” said Sen and colleagues in their publication. Human clinical trials are required to further assess whether the people will display the same results that were seen in aged mice upon urolithin A supplementation. As a result, urolithin A may provide a means to successfully target age-related sarcopenia.