Uncontrollably growing cells can form tumors, and when tumors form, they are confronted and invaded by the body’s defense–the immune system. Yet tumors often disarm our protective immune cells, which renders them ineffective and eliminates their cancer cell killing capabilities. This is true for the deadly and highly incurable brain cancer called glioblastoma. So, finding ways to reactivate our immune cells to eliminate tumors may help fight cancers like glioblastoma.
Sarkar and colleagues from the University of Calgary published an article in Science Translational Medicine showing that immune cells can be rejuvenated and reactivated by niacin (vitamin B3). The investigators showed that exposing these immune cells to niacin can inhibit the human cancer cell growth that initiates glioblastomas. “We highlight niacin, a common vitamin that can be quickly translated into clinical application, as an immune stimulator against glioblastomas,” said the investigators in the article.
Glioblastomas are the most common and most dangerous form of tumors that arise in the adult central nervous system. They are deadly, with a survival of about 15 months even when patients are treated with aggressive surgery, chemotherapy, and radiation. The prognosis for glioblastomas remains dismal, in part, because their stem-like cells — brain tumor-initiating cells — are relatively resistant to chemotherapy and radiation. So, there is a need to therapeutic development that targets brain tumor-initiating cells.
An additional feature that contributing to the deadly capabilities of glioblastomas is the tumors’ efficient exploitation of their surroundings, including the tumor-infiltrating immune cells. Immune cells that infiltrate tissues and tumors called macrophages and their blood circulating precursors called monocytes, collectively referred to as myeloid cells, may at first attempt to control tumor growth but are ultimately subverted by brain tumor-initiating cells and their progenies to repress the immune system and for the promotion of glioblastoma growth. Hence, medications that reactivate the infiltrating and fighting properties of compromised myeloid cells are highly desired for glioblastomas.
A recent study testing for molecules that affect myeloid cell activity showed that the common vitamin niacin had stimulatory effects. Niacin acts as a precursor to and has the ability to increase the levels of nicotinamide adenine dinucleotide (NAD+), a molecule playing critical roles in cellular energy generation that can also boost immune function. Niacin can increase human NAD+ in blood and potentially other tissues as well. Since niacin has been safely used in humans at high doses, Sarkar and colleagues investigated if niacin-stimulated myeloid cells would inhibit brain tumor-initiating cell growth of cells taken from glioblastoma patients.
To do so, the research team from the University of Calgary implanted glioblastoma tumor-initiating cells from patients into the brains of mice to form brain tumors. A week after the operation, they began to treat the mice with saline or niacin for 5 weeks, and 6 weeks after the implantation, they used brain imaging to examine the size of the brain tumors. The brain imaging showed that mice treated with niacin had brain tumors that grew to less than half the size of the brain tumors in mice treated with saline. Also, the niacin-treated mice ended up living longer compared to saline-treated mice.
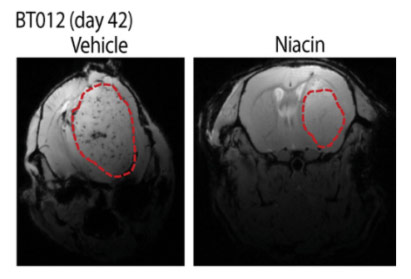
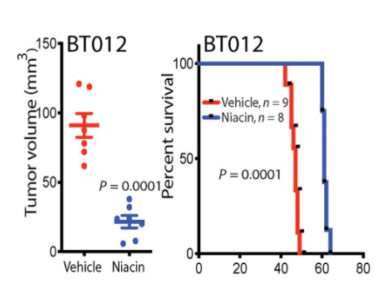
(Sarkar et al., 2020 | Science Translational Medicine) Niacin reduces the growth of patient-derived brain tumor-initiating cell implants in mice and prolongs their life span. Mice implanted with patient-derived glioblastoma cells in their brains were treated with niacin (100 mg/kg) or vehicle (saline) daily a week after the operation. The investigators assessed the therapeutic efficacy of niacin 6 weeks after implantation. The investigators observed large tumors in vehicle-treated mice and a much reduced tumor volume in those treated with niacin. Mice that received saline became symptomatic shortly thereafter, and survival plots showed that niacin prolonged lifespan.
The investigators then repeated these experiments, but this time they threw the current drug standard of care for glioblastoma, temozolomide, into the experiment. When they treated these mice with a combination of niacin and temozolomide, the mice survived longer than with either treatment alone. These experiments highlight the potential of adding niacin to the current therapeutic regimen for glioblastoma.
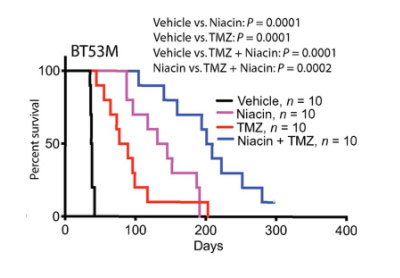
(Sarkar et al., 2020 | Science Translational Medicine) Temozolomide promotes the life-extending efficacy of niacin in mice with patient-derived brain tumor-initiating cells. Temozolomide (TMZ) improved survival, which was exceeded in the niacin group. Notably, the combination of niacin and temozolomide substantially prolonged survival by at least five times compared to untreated mice (vehicle); the combination also conferred a survival advantage when compared to niacin.
The investigators found that niacin-exposed monocytes attenuated the growth of brain tumor-initiating cells derived from glioblastoma patients by secreting a molecule called interferon-α14 that inhibits the growth and replication of cells that have its receptor, which is often found on cancer cells. Along these lines, the therapeutic effects of niacin were negated in mice harboring brain tumor-initiating cells lacking the receptor for interferon-α14, which showed that niacin’s effects were dependent on interferon-α14.
They then wanted to see if niacin could elicit the same effect with human myeloid cells. To do so, the investigators took myeloid cells from glioblastoma patients as well as people without glioblastomas and cultured them in petri dishes. They then took the solutions that these myeloid cells were grown in and tried to grow brain tumor-initiating cells from different glioblastoma patients.
The solutions from myeloid cells taken from healthy patients inhibited the brain tumor-initiating cells’ growth whereas the solutions from myeloid cells taken from glioblastoma patients had no effect. However, when they repeated the experiment but this time added niacin to the myeloid cells from glioblastoma patients, the investigators saw a major decrease in the growth of brain tumor-initiating cells. This shows that niacin treatment reactivated the brain tumor-initiating cell growth inhibitory ability of glioblastoma patient-derived monocytes.
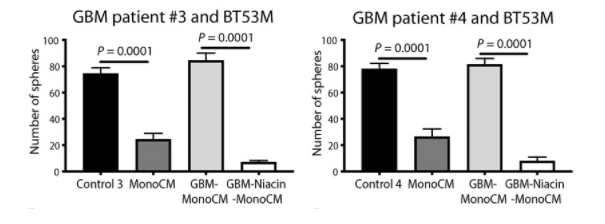
(Sarkar et al., 2020 | Science Translational Medicine) Monocytes from glioblastoma patients do not suppress brain tumor-initiating cell growth in culture but are reactivated by exposure to niacin. Brain tumor-initiating cells were exposed to solutions from monocytes either from healthy volunteers (MonoCM) or glioblastoma patients (GBM-MonoCM). Unlike cells from healthy human volunteers, monocytes isolated from the blood of glioblastoma patients did not inhibit the growth of human brain tumor-initiating cells. Notably, niacin treated glioblastoma-patient derived monocytes (GBM-Niacin-MonoCM) restored their capacity to inhibit the growth of brain tumor-initiating cells.
Notably, niacin increased the amount of the anti-proliferation factor interferon-α14 in the solutions from glioblastoma-derived myeloid cells. When the investigators blocked the receptor for interferon-α14, niacin no longer reactivated the tumor growth-inhibiting capacity of glioblastoma patient-derived monocytes, thus supporting the involvement of interferon-α14 in niacin-induced tumor control.
These findings show that niacin is a promising treatment for the currently incurable glioblastomas. “We propose the immune-stimulatory activity of niacin, a common vitamin that could be rapidly translated into clinical use, as an adjunctive treatment for patients with glioblastoma,” said the investigators in the article. “The subverted myeloid immunity in other cancers could also be conducive for niacin intervention, but this remains to be determined in future studies.”