Key Points:
- Preliminary results show that whole-brain NAD+ levels decline with age in healthy subjects, confirming previous studies of the occipital lobes — where vision is processed.
- In the future, the methods employed in this study can be used to determine whether NAD+ boosting therapies can replenish NAD+ levels in the brains of middle-aged and older adults.
With vital roles in brain aging, cognitive function, and neurodegeneration, NAD+ (nicotinamide adenine dinucleotide) has instigated a flurry of intensive scientific investigation. On the premise that brain NAD+ levels decline with age, NAD+ replenishing therapies could counteract the cellular dysfunction underlying the brain’s time-dependent structural and functional deterioration.
However, non-invasively quantifying NAD+ levels beneath the skull is challenging, as the minute concentrations of NAD+ within the brain’s cells limit its detection to small regions. Despite recent advances in magnetic resonance (MR) technology, quantifying whole-brain intracellular NAD+ levels remains a technical obstacle.
Now, researchers from the University of Minnesota have devised a method for generating a whole-brain map of NAD+ in humans. Furthermore, their preliminary analysis helps to confirm that NAD+ levels decline with age in the brain. Still, these results are preliminary, as the majority of subjects were below 30 years old, two subjects were 34 years old, and only one subject was over the age of 60.
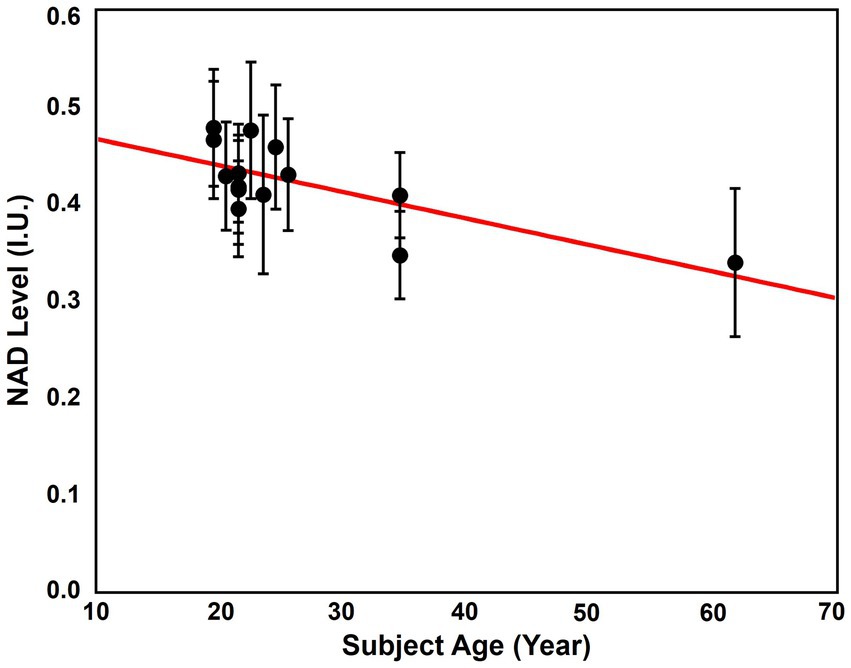
The first study to show that NAD+ declines with age in the human brain was limited to the occipital lobe — the vision processing center of our brain. A subsequent study also showed that NAD+ declined in the occipital lobe, confirming the findings of the first study. Building on this, the results of the University of Minnesota study suggest that NAD+ levels (0.4 mM) are the same across the entire brain and decline with age.
Replenishing NAD+ Levels with Age
In addition to NAD+ levels dropping in the human brain, it has been shown to decline in other organs and organ systems, such as the liver, skin, and immune system. Furthermore, animal studies suggest that NAD+ levels drop with age in nearly every organ, including our muscles and heart. Since NAD+ facilitates the production of cellular energy, the small molecule is essential life on a cellular level.
Importantly, the dysregulation of cellular energy metabolism underpins chronic age-related diseases, including dementia, cardiovascular disease, and cancer. These diseases are also associated with deficits in NAD+ metabolism, which plays a vital role in cellular energy production. For this reason, scientists hypothesize that replenishing NAD+ levels with age can counteract chronic age-related diseases, thereby potentially prolonging the lifespan of many individuals.
Diet and Exercise
Studies show that obesity drives metabolic deficits that can lead to lower NAD+ levels. Therefore, NAD+ levels could be maintained by minimizing the intake of calorically dense foods lacking sufficient vitamins and minerals, including:
- Soda containing high levels of sugar
- Alcohol, especially beer and mixed drinks (e.g. margaritas)
- Fast food
- Candies
- Baked goods
- Ice cream
- Pizza
Importantly, exercise can also counteract obesity while raising NAD+ levels, suggesting that withholding from high-calorie foods and adhering to habitual physical activity can maintain or replenish NAD+ levels.
NAD+ Precursors
NAD+ precursors are molecules that are converted to NAD+ in the body. Supplementing with NMN, a popular NAD+ precursor, has been shown to raise blood NAD+ levels in healthy adults. Furthermore, NMN shows promise in counteracting the metabolic dysregulation associated with a higher-than-normal body mass index (BMI) and the decrements in physical performance associated with older age. Moreover, intravenous (IV) NAD+ has been shown to relieve symptoms of addiction, depression, and neurodegenerative disease, suggesting NAD+ precursors could do the same.
However, whether NAD+ precursors increase NAD+ levels in the human brain is unknown. Now, by using the latest methods employed by the University of Minnesota researchers, it is possible to measure changes in whole-brain NAD+ levels after supplementing with NAD+ precursors. Therefore, future studies using this method can confirm whether NMN or NR can replenish NAD+ levels in the brains of individuals over the age of 30.
How Is NAD+ Measured in the Brain?
To measure brain NAD+ levels, the University of Minnesota researchers employed phosphorus-31 magnetic resonance spectroscopic imaging (31P-MRSI). Like magnetic resonance imaging (MRI), 31P-MRSI utilizes a large magnet that surrounds the head of subjects. However, unlike MRI, which exploits hydrogen atoms in water, 31P-MRSI exploits the phosphorus atoms found within various molecules, including NAD+.
Phosphorus-31 (31P) has intrinsic magnetic properties that can be manipulated by an external magnetic field. Hence, MRI machines generate a powerful magnetic field that aligns 31P atoms and causes them to gyrate at a specific frequency. The 31P atoms are then rotated using radio waves of the same frequency — the resonance frequency. This rotation of 31P atoms releases an electrical current that can then be detected and read as a signal on a computer interface.
The University of Minnesota researchers used a 7 T MRI machine. “T,” which stands for Tesla, named after the great inventor Nikola Tesla, denotes the strength of the magnetic field. The higher the strength of the magnetic field, the better the resolution of the signal produced by the MRI machine. There are only 100 7 T MRI machines in the world. For comparison, there are about 20,000 3 T machines in the world.
Depending on the molecule they are a part of, 31P atoms gyrate at different frequencies. For example, 31P atoms in NAD+ gyrate at a different frequency than the 31P atoms in ATP (adenosine triphosphate) — cellular energy. The signal produced by MRI machines is a spectrum of frequencies that reflect the concentration of different 31P-containing molecules. As such, the researchers used a new processing method to resolve the signal for NAD+.
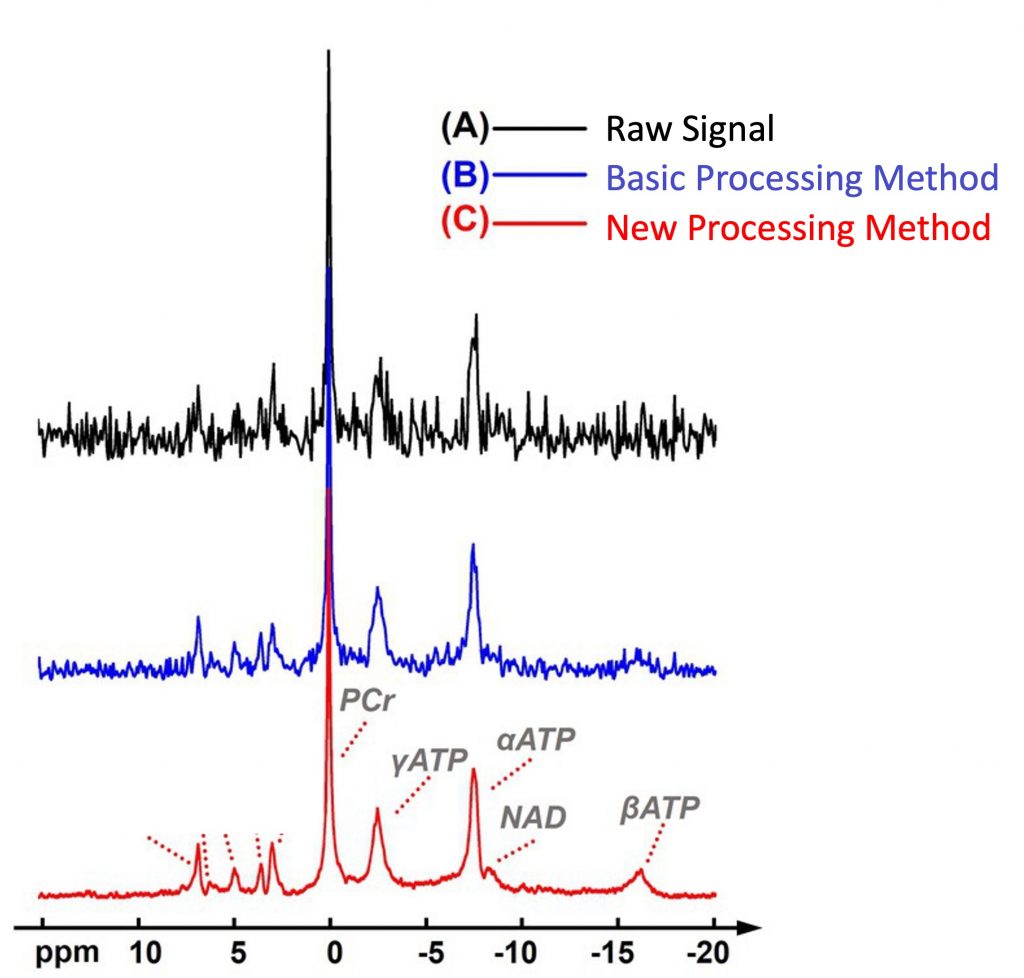
By calculating the concentration of NAD+ based on their new processing method, the researchers determined the levels of NAD+ across the entire brain. The accuracy of their measurements was confirmed with computer simulations and previous measurements.
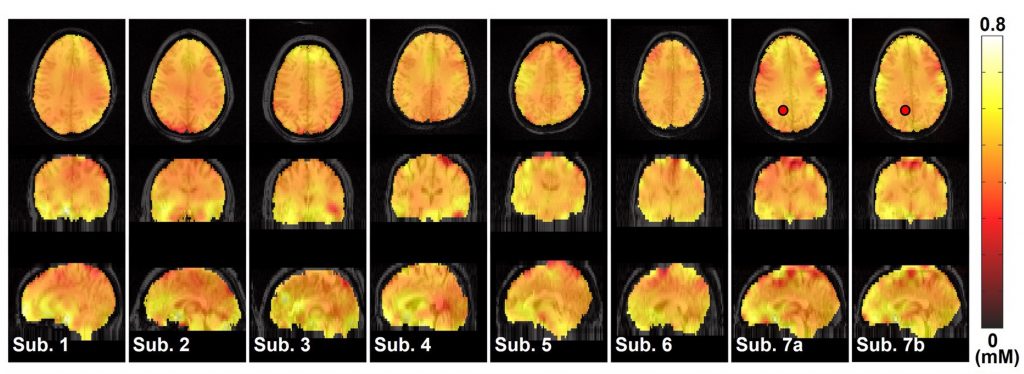
With NAD+ brain scans, we may be able to determine whether low NAD+ levels play a causal role in neurological conditions like depression. For example, a recent mouse study showed that low NAD+ in a region of the brain called the frontal cortex was associated with depressive behavior. Hence, patients who are unresponsive to traditional anti-depressants could receive a brain scan to determine if they have low frontal cortex NAD+ levels. If so, they could be treated with NAD+ precursors like NMN. Still, more studies are needed to determine the role of low NAD+ levels in specific brain regions and how this affects psychiatric conditions, dementia, and neurodegeneration.