Key Points:
- A freshly developed drug called VBIT-4 prevents learning and memory deficits in Alzheimer’s mice.
- VBIT-4 stops mitochondria-associated neuron death, indicating the prevention of neurodegeneration.
- Inflammation of the brain (neuroinflammation), implicated in triggering Alzheimer’s, is prevented by VBIT-4.
Someone develops Alzheimer’s disease (AD) every 66 seconds in the United States. However, we still don’t know what triggers this neurodegenerative disease. Now, researchers from the Ben-Gurion University of the Negev in Israel have clued in on a protein called VDAC1. VDAC1 is embedded within the walls of mitochondria and transports a multitude of molecules, including NAD+. Moreover, VDAC1 has the power to evoke inflammation and programmed cell death — apoptosis.
As reported in Translational Neurodegeneration, Verma and colleagues demonstrate that targeting VDAC1 with a drug called VBIT-4 prevents many of the symptoms and brain dysfunction associated with AD. By placing 20 mg/kg of VBIT-4 in the drinking water of AD mice, the Israeli scientists were able to prevent memory loss and neurodegeneration, the most prominent features of AD. They also prevented neuroinflammation with VBIT-4, pointing to inflammation as an AD trigger.
Inhibiting Mitochondrial Dysfunction Prevents Alzheimer’s Symptoms
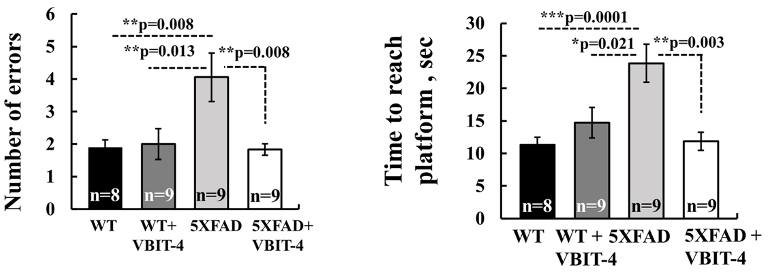
To test the effects of VBIT-4, Verma and colleagues gave 20 mg/kg of the drug to 5xFAD mice, a well-studied mouse model for AD. The AD mice were then subjected to a series of behavioral tests to assess their learning and memory. One of these tests called the radial arm water maze, involved the mice learning to navigate and remember the location of an escape platform.
During the maze test, AD mice that did not receive VBIT-4 made nearly twice as many errors and took twice as long to find the platform as normal mice. However, AD mice given VBIT-4 did just as well as normal mice on the test. These findings suggest that treatment with VBIT-4 prevents learning and memory deficiencies associated with AD mice.
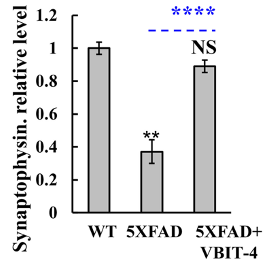
Once the behavioral tests were completed, Verma and colleagues examined the mice’s brains for signs of neurodegeneration. To conduct this examination, Verma and colleagues measured a protein called synaptophysin, which is only found in neurons. They found a 3-fold decrease in synaptophysin in the brains of untreated AD mice. In contrast, AD mice treated with VBIT-4 did not exhibit a reduction in this protein, suggesting the prevention of neurodegeneration.
VBIT-4 works by preventing VDAC1 from forming a large channel within the outer wall of mitochondria. It is this large hole that allows various factors to be released from the mitochondria and activate inflammation and apoptosis. While Verma and colleagues showed that apoptosis and neuron loss was mitigated by VBIT-4, they further investigated whether VBIT-4 could prevent inflammation.
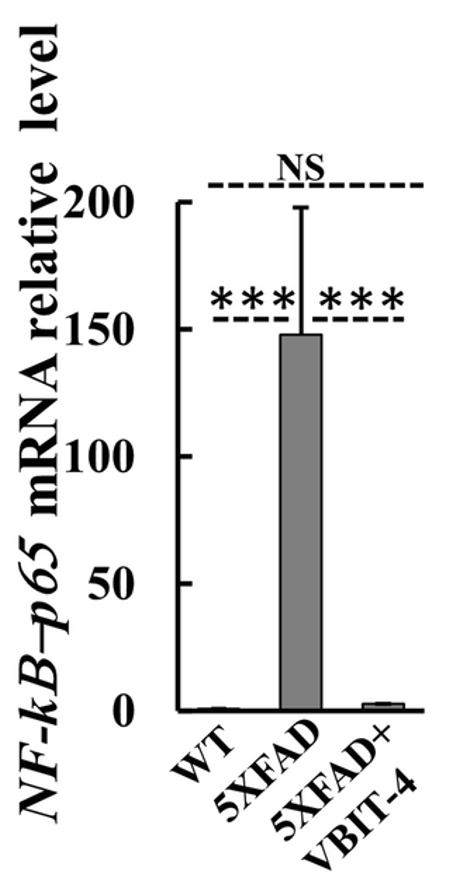
To assess inflammation, the researchers measured various factors, including a protein called NF-𝜅B-p65. They found that the mRNA for NF-𝜅B-p65 was dramatically increased in the brains of untreated AD mice. However, AD mice treated with VBIT-4 did not display this increase. These findings suggest that VBIT-4 treatment can prevent features of neuroinflammation in AD mice.
Targeting Mitochondrial Health to Counter Aging
VBIT-4: A Future Anti-Aging Drug?
Overall, the findings of Verma and colleagues suggest that VBIT-4 prevents much of the symptoms and brain pathology associated with AD by preventing VDAC1 proteins from forming an apoptosis and inflammation-mediating channel in mitochondria. If held up in future animal and human studies, these findings could revolutionize the way AD patients are treated.
Furthermore, Verma and colleagues have previously shown that VBIT-4, and another one of their drugs called VBIT-12 prevent mitochondrial dysfunction and apoptosis in mouse models for type 2 diabetes, lupus, and colitis. This could mean that these VBIT drugs, targeting the so-called gatekeeper of mitochondria, VDAC1, could potentially treat these diseases in the future if these findings can be translated to humans.
Current Interventions for Targeting Mitochondrial Health
Mitochondrial dysfunction is a hallmark of aging, meaning that it underlies several chronic age-related conditions, including neurodegeneration. Our mitochondria produce cellular energy and are thus critical for cell survival and ultimately organ and tissue function. As we age, our mitochondria tend to become less efficient, leading to their dysfunction.
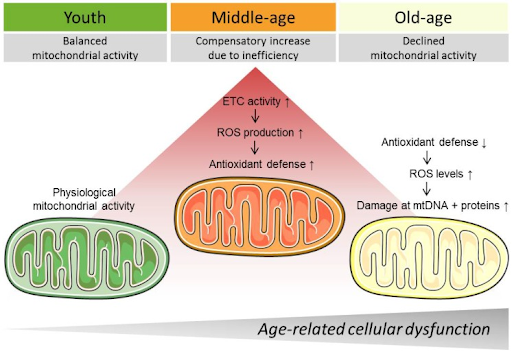
It may be possible to slow the deterioration of our mitochondria with several strategies, including diet and exercise. For example, caloric restriction — consuming fewer calories by eating less food — has been shown to increase the energy produced by mitochondria. Furthermore, exercise has been shown to improve muscle mitochondrial health, which may aid in countering the age-related decline in muscle strength and size — sarcopenia. Pharmacological interventions that have been shown to improve mitochondrial health or counter ROS include senolytics, NAD+ precursors, and polyphenols like resveratrol.