Key Points:
- Aging cells lose vital epigenetic instructions needed to work properly in our organs, which slows down cell and organ function.
- The age-related loss of intact epigenetic information can be retrieved to improve the function of damaged and aged tissues, potentially catalyzing age reversal.
Information storage and retrieval is not only a cornerstone of technology like telecommunications and the internet but also a fundamental aspect of life. Every complex organism, from plants to humans, relies on the intricate information encoded in DNA. This DNA acts as a blueprint for protein production, essential for all biological functions. Beyond the DNA blueprint lies a complex layer of regulation known as epigenetics, which controls how this genetic information is expressed to form different tissues and organs in the body.
DNA tagging patterns play a crucial role in this epigenetic regulation. These patterns are created by adding small chemical markers either directly onto the DNA or onto histones, the proteins around which DNA winds to fit within a cell’s nucleus. These chemical tags can activate or deactivate genes, thereby influencing protein production and cell function. The state of these epigenetic tags is dynamic and can be altered by various factors, including environmental influences, cellular damage, and the aging process. Like scratches on a CD, these epigenetic changes can accumulate over time, leading to decreased fidelity in gene regulation, similar to playback errors when listening to music from damaged CDs.
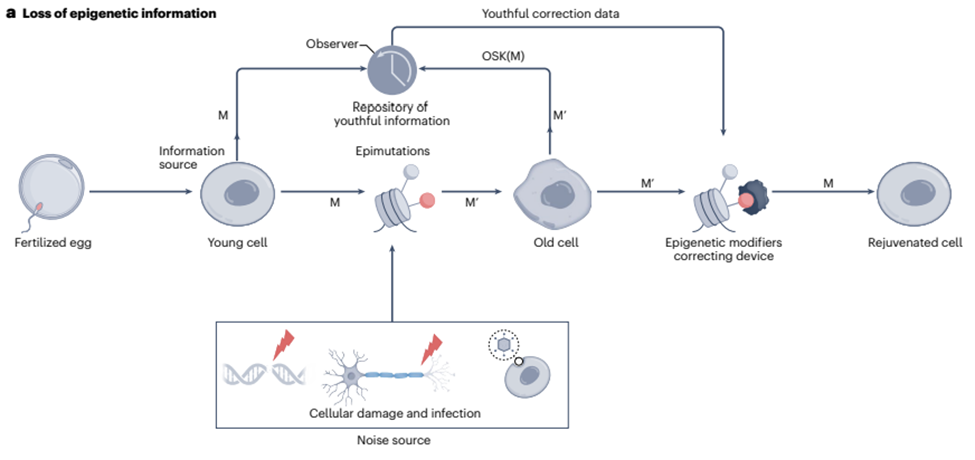
A review by Sinclair and colleagues from Harvard Medical School, published in Nature Aging, details the Information Theory of Aging (ITOA). This theory suggests that the loss of intact, youthful epigenetic information is a driving force behind the aging process. It proposes that it is possible to retrieve and restore this lost information through a process known as epigenetic reprogramming. This involves activating a set of genes called Yamanaka factors, which have the potential to rejuvenate cells. This theory, if supported by future research, could revolutionize our understanding of aging and pave the way for novel anti-aging therapies.
Youthful Epigenetic Information: The Key to Cellular Identity and Function
At the cellular level, every cell in our body contains the same DNA, but it is epigenetic information that dictates which genes are turned on or off, thus determining the cell’s function and identity. For example, a neuron in the brain follows neuron-specific instructions encoded in its DNA, guided by its unique epigenetic pattern. However, as we age, the loss of this epigenetic information can lead to a loss of cellular identity, impairing tissue function and contributing to the aging process.
Recent research has shown that older cells retain a form of youthful epigenetic information, which can be reactivated through epigenetic reprogramming. Sinclair and his team demonstrated this by using Yamanaka factor genes to rejuvenate neurons in aged mice, leading to restored vision. This technique also extended the lifespan of mice with a premature aging condition by about 40%.
However, the activation of Yamanaka factor genes must be carefully controlled. Prolonged activation can lead to cells losing their identity entirely, reverting to a stem cell-like state. This can result in excessive cell proliferation and potentially lead to cancer. Therefore, the challenge lies in retrieving youthful epigenetic information without compromising cellular identity, offering rejuvenation benefits while minimizing cancer risks.
Exploring the Mysteries of Epigenetic Backup Copies
A pivotal assumption of the ITOA is the existence of a backup copy of youthful epigenetic information within cells, which can be restored to improve the function of aged tissues. The exact mechanisms of how this backup copy is stored and maintained are still under investigation.
Sinclair’s team suggests that certain genetic mechanisms act as either passive or active observers of the epigenetic landscape that can restore intact epigenetic information. Passive observers, such as specific DNA sequences called CpG islands, can be marked during development to establish cellular identity and typically remain unchanged during aging. Conversely, active observers such as histone tags undergo frequent changes, and the global pattern of tagging may shift with age. Through altering active observers, epigenetic reprogramming may restore intact epigenetic information. Active observers may thus help to restore a more youthful epigenetic code during reprogramming all the while gathering DNA location information from passive observers.
Research indicating that epigenetic age can be reversed, as measured with epigenetic aging clocks that read epigenetic information to evaluate biological age, supports the theory of a backup copy. Lower or higher biological ages represent decelerated or accelerated aging, respectively, and reversing biological age measured with epigenetic information suggests restoring an intact epigenetic code. Understanding where and how this backup code is stored could be crucial in developing new anti-aging strategies based on epigenetic information.
The study of epigenetics and its role in aging represents a new frontier in biology. As research continues to unveil the mysteries of how epigenetic information is stored, altered, and potentially restored, we move closer to understanding the complex processes of aging and developing interventions to improve health in our later years.