Key Points:
- Circadian clock malfunction hinders muscle repair by inhibiting the formation of new muscle cells after injury.
- Dysfunctional muscle formation is due to a shift from glucose to fat metabolism, reducing NAD+ levels.
- Boosting NAD+ restores SIRT1 gene modulation and muscle cell formation.
Molecular clocks control many of our body’s biological processes, from our sleeping patterns to the amount of oxygen we breathe. However, aging can throw our clocks out of sync, leading to the onset of conditions like diabetes, obesity, and cancer.
According to a new study, even muscle repair seems to adhere to these clocks. In the journal Genes & Development, Zhu and colleagues from Northwestern University report that muscle repair is dependent on circadian clocks. Following injury, greater muscle repair capacity occurs when mice are more active and awake than when they are inactive and resting. But when the molecular clocks behind muscle repair fail, the formation of new muscle cells necessary for muscle regeneration goes awry due to muscle stem cells shifting from glucose to fat utilization, leading to low NAD+ – a critical bioenergetic molecule – levels. Boosting NAD+ restores muscle cell formation by making up for this failed shift in metabolism.
“Our finding that restoring the redox metabolite, NAD+, to clock-disrupted stem cells can restore normal proliferation and muscle fiber formation is exciting because it suggests that NAD+ restoration can counteract the effects of muscle injury at the ‘wrong’ time of day, during normal resting hours,” said Clara Peek, Ph.D., Assistant Professor of Biochemistry and Molecular Genetics at Northwestern University
The Muscle Repair Process
Muscle repair (regeneration) involves muscle stem cells multiplying and fusing to become muscle cells, the functional units of muscle. When a muscle is injured, blood vessels often rupture and cause a lack of oxygen (hypoxia), reactivating muscle stem cells to begin the muscle repair process. Activated muscle stem cells are called myoblasts, the precursors to fully grown muscle cells. Activated myoblasts propagate – divide and multiply to increase in number – then fuse to form a tube. The fused tube (myotube) becomes a mature muscle cell in the differentiation process, completing the muscle repair process. Little is known about how circadian clocks control muscle repair.
Muscle Repair Depends on Molecular Clocks
Circadian clocks can’t be wound up and set like a mechanical clock but are regulated by genes that are the master switches for activating other genes. These master switches are called Bmal1 and Clock, and they direct the timing of specific functions within a given type of cell. Zhu and colleagues began investigating the molecular muscle clock because muscle repair was higher during the active/wake period than in mice’s inactive/rest period.
To determine if clock-dependent muscle repair is specific to muscle stem cells, Zhu and colleagues used genetically modified mice with reduced Bmal1 in their muscle stem cells. The results showed that after muscle injury, muscle stem cells without Bmal1 had signs of impaired muscle repair, demonstrating that muscle stem cell clocks are necessary for the muscle repair process.
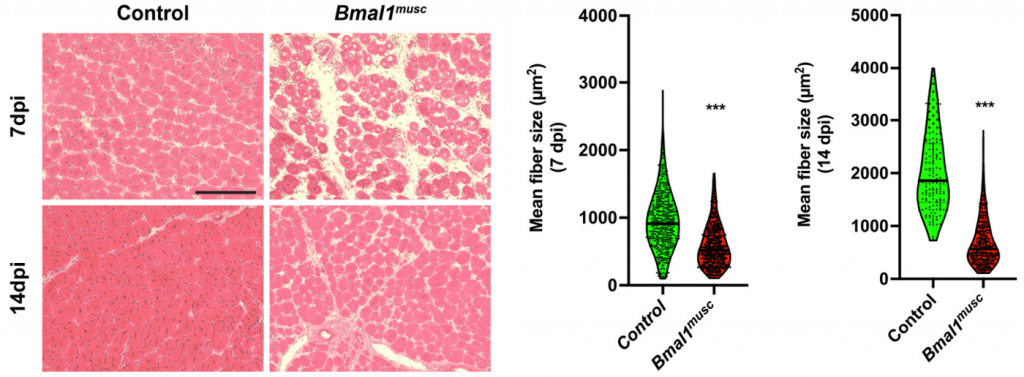
(Zhu et al., 2022 | Genes & Development) Muscle Stem Cell Clock Controls Muscle Repair. 7- and 14-days post-injury (dpi), mice with reduced clock gene Bmal1 in their muscle stem cells (Bmal1musc) display impaired muscle repair as shown by smaller muscle cell (fiber) size. (left) Representative images of muscle slices from control and Bmal1musc. (right) Quantification of muscle size.
Molecular Clock Regulates Proper Muscle Formation
To investigate the role of myoblast molecular clocks during hypoxia, Zhu and colleagues used myoblasts with Bmal1 deleted. They showed that these myoblasts failed to propagate and differentiate during hypoxia. Additionally, gene analysis revealed decreased levels of propagation genes in Bmal1-deficient myoblasts but increased levels of differentiation genes, which was unexpected because the myoblasts failed to differentiate. These results indicate that although Bmal1 loss leads to activating genes associated with muscle differentiation, this is insufficient to drive proper muscle cell formation.
Loss of Clock Changes Choice of Nutrient Utilization
Both gene analysis and direct measurements showed that myoblasts lacking Bmal1 switch from metabolizing glucose – glycolysis – to metabolizing fat. As part of impaired glycolysis, Bmal1-deficient myoblasts had decreased lactate production, which was worse under hypoxic conditions. When lactate is produced, NAD+ is regenerated to sustain glycolysis. Therefore, Zhu and colleagues hypothesized that reduced lactate production would decrease NAD+. Indeed, they observed lower NAD+ levels in myoblasts lacking Bmal1, suggesting that impaired glycolysis leads to reduced NAD+.
Low NAD+ Levels Alter Gene Activation through SIRT1
Since NAD+ feeds the activity of sirtuin 1 (SIRT1), an enzyme that modulates the activation of genes through DNA accessibility (epigenetic regulation), Zhu and colleagues assessed DNA accessibility in Bmal1-/- myoblasts. They found an overall increase in DNA accessibility to genes associated with differentiation. Since SIRT1 decreases DNA accessibility, these findings suggest that decreased NAD+-dependent SIRT1 activity gives access to differentiation genes Bmal1-/- myoblasts.
NAD+ Restores Muscle Clock Defects
Zhu and colleagues tested whether NMN (nicotinamide mononucleotide), a precursor to NAD+ that increases NAD+ levels, could rescue the effects of Bmal1 loss. They found that NMN has a partial impact, suggesting that NAD+ deficiency underlies the propagation and differentiation defects in myoblasts lacking Bmal1. To help confirm this, the Northwestern scientists treated the Bmal1-deficient myoblasts with NADH oxidase (via lentivirus), an enzyme that increases NAD+. They found that boosting NAD+ restored myoblast propagation and differentiation during postinjury hypoxia. Boosting NAD+ was also linked to reduced DNA accessibility to differentiation genes, suggesting that SIRT1 activity was restored. These findings show that NAD+ can restore the muscle clock defects observed in clock-corrupted myoblasts.

(Zhu et al., 2022 | Genes & Development) Boosting NAD+ Restores Muscle Stem Cell Defects. An empty (EV) or NADH oxidase containing (LbNOX) lentiviral vector was delivered to control (WT) and Bmal1-/- (KO) myoblasts. (left) Representative images of myoblasts in all conditions. (middle) Propagation (growth rate) and (right) differentiation of Bmal1-/- myoblasts were increased by boosting NAD+.
Could NAD+ Reprogram our Muscles in Old Age?
Zhu and colleagues reveal a novel role for the molecular muscle clock in muscle repair, which could have implications for muscle regeneration in old age. Many older adults cannot gain muscle mass and strength despite resistance exercise training. This may be due to a lack of muscle regenerative capacity resulting from improper muscle stem cell activation. Since our molecular clocks become dysregulated with age, it may be that our muscle stem cell clocks contribute to muscle mass and strength loss in old age. If so, therapies like boosting NAD+ could help to reprogram the genes responsible for proper stem cell activation and muscle cell formation after resistance exercise.