Key Points:
- Inhibiting the signaling molecule IL-11 prolongs the lifespan of male and female mice by 22.3%.
- IL-11 inhibition also reduces cancer incidence and improves body composition and strength.
- Blocking IL-11 has similar effects to metformin and rapamycin.
Scientists from Duke–National University of Singapore Medical School have significantly increased the lifespan of mice by injecting them with an antibody that inhibits a protein called interleukin-11 (IL-11).
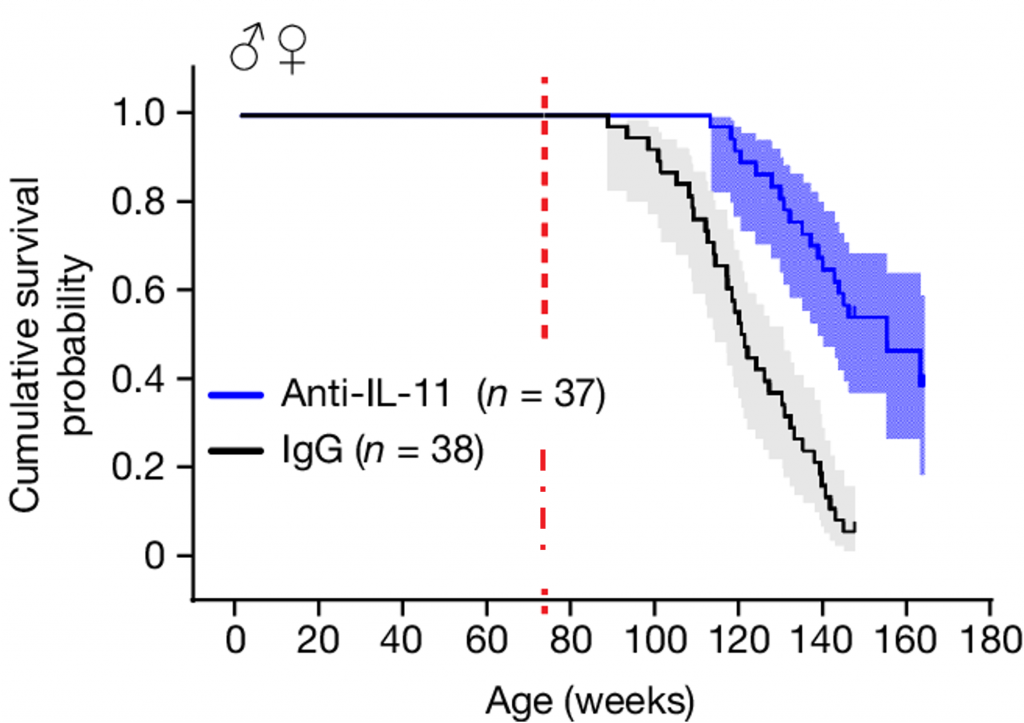
In the mice that lived longer, autopsies revealed a lower incidence of tumors, suggesting that inhibiting IL-11 may delay or prevent cancer development. Furthermore, inhibiting IL-11 was shown to reduce fat mass, increase lean mass, boost full-body strength, and prevent frailty, a measure of overall health. Together, these findings demonstrate that inhibiting IL-11 can prolong lifespan and improve healthspan — the duration of life lived in good health.
Inhibiting IL-11 Blocks Multiple Key Drivers of Aging
Until recently, IL-11 was largely misunderstood by scientists. The protein was once thought to have anti-inflammatory, anti-scarring, and pro-regenerative properties. However, scientists now believe it possesses opposite properties and is pro-inflammatory, pro-scarring, and anti-regenerative.
Still, IL-11 is a powerful signaling molecule that modulates the master nutrient sensors AMPK and mTOR. AMPK is activated during nutrient deprivation, such as when we are in a fasted state. When activated, AMPK triggers cell survival programs that protect against cellular aging, including the inhibition of mTOR. For this reason, the anti-diabetes drug metformin, which activates AMPK, was popularized as one of the first repurposed anti-aging drugs.
An even more promising repurposed anti-aging candidate is the immune-modulating drug rapamycin. Rapamycin inhibits mTOR (mammalian target of rapamycin) and has repeatedly been shown to robustly increase the lifespan of mice. Importantly, the Duke researchers found that inhibiting IL-11 activates AMPK (similar to metformin) and inhibits mTOR (similar to rapamycin).
Furthermore, the researchers showed that IL-11 inhibition largely prevents the age-related increase in senescent cells. Senescent cells are thought to exacerbate chronic low-grade inflammation, referred to as inflammaging because it underlies many chronic age-related diseases. Thus, inhibiting IL-11 alleviates multiple key drivers of aging, including deregulated nutrient sensing, cellular senescence, and chronic inflammation.
An IL-11 Antibody for Humans
Previously, higher levels of IL-11 were observed in older individuals, suggesting the protein increases with age in our bodies. To help confirm this, Duke researchers demonstrated that IL-11 increases with age in the liver, muscle, and visceral fat of mice. However, whether inhibiting IL-11 in older adults is safe remains an open question.
Scientists like Charles Brenner, Ph.D., have pointed out that IL-11 is an important component of the immune system. Laboratory mice are kept in a pathogen-free environment, largely protecting them from infections. If blocking IL-11 in humans suppresses the immune system, which is already weakened in older individuals, it could lead to a higher incidence of infection and illness. Therefore, studies will be needed to determine how inhibiting IL-11 in humans affects the immune system.
As it turns out, we may soon know if inhibiting IL-11 is safe for humans. The German-based pharmaceutical company Boehringer Ingelheim has begun a Phase I clinical trial to test the safety and tolerability of an IL-11 inhibitor antibody in healthy volunteers. If successful, the IL-11 inhibitor will go on to be tested for treating fibrosis — scarring of the organs and tissues. Fibrotic diseases, which can trigger organ failure and cancer, accounted for 35% of global deaths in 2019. This means that if inhibiting IL-11 prevents fibrosis, many individuals worldwide could live longer.
The Duke researchers conclude,
“Our data suggest that anti-IL-11 therapy, which has a reassuring safety profile and is currently in early-stage clinical trials for fibroinflammatory diseases, is a potentially translatable approach for extending human healthspan and lifespan.”