In general, the solution to treat patients that exhibit end-stage organ failure is organ transplantation. While the supply and demand gap for organ transplantation is immense, there is potential in bridging this gap through the use of older organs. However, older organs tend not to be utilized in transplantation due to inducing elevated immune responses and increased senescence, a state of cell arrest that prevents cellular growth and division.
In an article published in Nature Communications, Iske and colleagues from Harvard Medical School evaluated how senolytics—compounds that eliminate senescent cells—affect heart transplants from aged donors in mice. They demonstrate that senolytics diminish age-specific immune responses and extend the survival of aged doner cardiac transplants similar to youthful donor organs.
“Our data provide rationale for considering clinical trials treating donors, organs, or recipients with senolytic drugs to optimize the use of organs from older donors, helping to close the gap between organ availability and the needs of the many patients currently on transplant waiting lists,” concluded the authors of this article.
Why do old organ transplants fail?
Utilizing aged donors for transplantation increases the chance of unfavorable outcomes, such as higher recurrence of kidney or heart transplant rejections. In addition, the recovery after transplantation-induced ischemia-reperfusion injury—tissue damage that occurs upon the return of blood supply to tissue (reperfusion) following a period of diminished oxygen levels (ischemia)—is hampered in older organs. Clinically, this correlates to increased rates of delayed graft function.
These negative transplant consequences may be linked to how aging is connected with elevated senescence that is related to chronic, low-grade, sterile inflammation called infamm-aging. Furthermore, a known contributor of diminished renal function and cardiac stress resilience is the buildup of senescent cells that occurs upon aging.
Ischemia-reperfusion injury is clinically relevant in a myriad of diseases, especially in the elderly. Comprehension of the underlying modifications in tissues during stress-surveillance responses is clinically important and extremely relevant to various diseases and conditions, including transplantation. The link between the build of senescent cells, transplantation results, and the potential for targeting cells to reduce damage and increase immunogenicity in aged organs has yet to be adequately investigated.
Old donor organs renewed with senolytics
According to Iske and colleagues, ischemia-reperfusion injury in mice caused a systemic rise in circulating mitochondrial DNA, a tiny circular chromosome located within the mitochondria that secretes out of injured, diseased, and terminal cells. They then demonstrated that this cell-free mitochondrial DNA induced age-specific inflammatory responses moderated by dendritic cells, immune cells that trace foreign molecules. Furthermore, the investigators traced the origins of this circulating mitochondrial to senescent cells, which accumulate with age and enhance the immune response to transplantation.
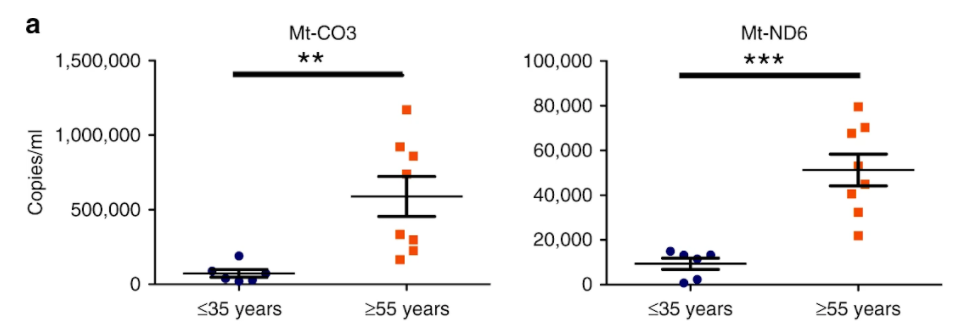
Iske and colleagues evaluated the clinical relevance of these results by comparing the concentrations of cell-free mitochondrial DNA in plasma samples of human organ donors older than 55 years old or younger than 35 years old. In particular, when compared to older donors, young donors frequently exhibited lower plasma concentrations of cell-free mitochondrial DNA. In addition, the investigators noted upregulation of the compounds essential for producing alloimmune responses after using cell-free mitochondrial DNA to activate human dendritic cells.
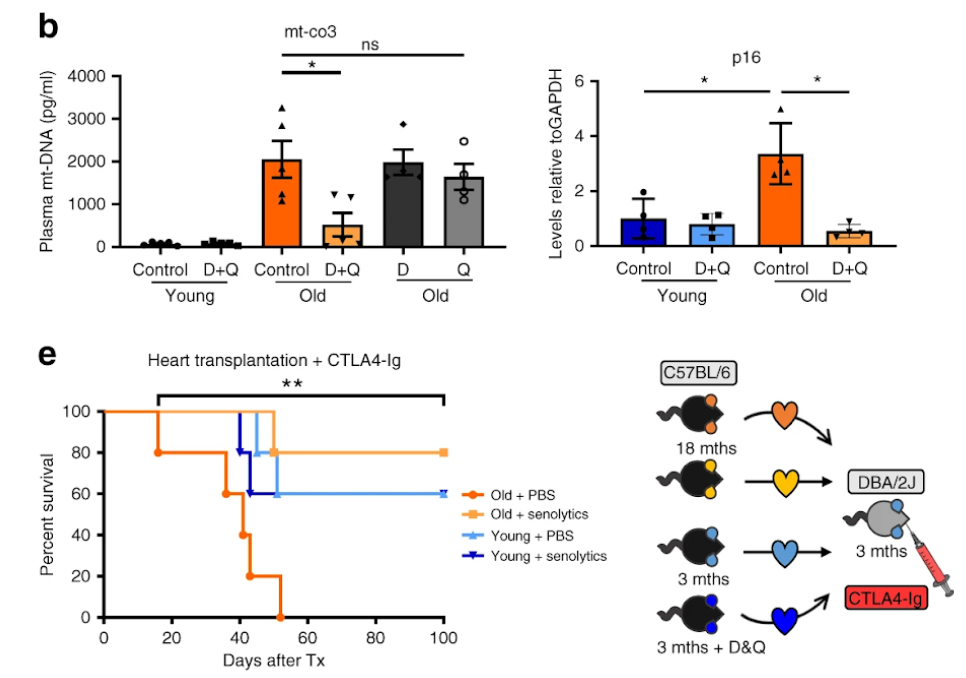
Iske and colleagues proceeded with examining the effects of two senolytics: the chemotherapeutic dasatinib and the natural, plant-based quercetin, which is present in various food such as Brassica vegetables, apples, grapes, berries, onions, and tomatoes, in addition, multiple seeds, nuts, barks, flowers, and leaves. The investigators detected diminished senescent cells and reduced secretion of cell-free mitochondrial DNA in rodents administered with both the senolytics dasatinib and quercetin. As a result, age-specific immune responses appeared to be suppressed. Notably, treatment with either dasatinib or quercetin alone was less efficient in lowering circulating mitochondrial DNA levels than the combination of both senolytics.
Critically, therapy with dasatinib and quercetin increased the longevity of cardiac tissue grafts from elderly donors to levels similar to those seen in young donors. Furthermore, the majority of hearts from treated elderly donors (80%) survived the monitoring period (100 days), whereas organs from untreated elderly donors stopped operating after 37 days. This suggests that cellular senescence-induced organ failure, inflammation, and dysfunction associated with transplanting organs from older donors could be reduced using these senolytic medicines in the future.
“Collectively, we identify accumulating cell-free mitochondrial DNA as a key factor in infamm-aging and present senolytics as a potential approach to improve transplant outcomes and availability,” concluded the authors of this article. These findings highlight the clinical potential of senolytics for allowing the transplantation of organs from elderly donors.